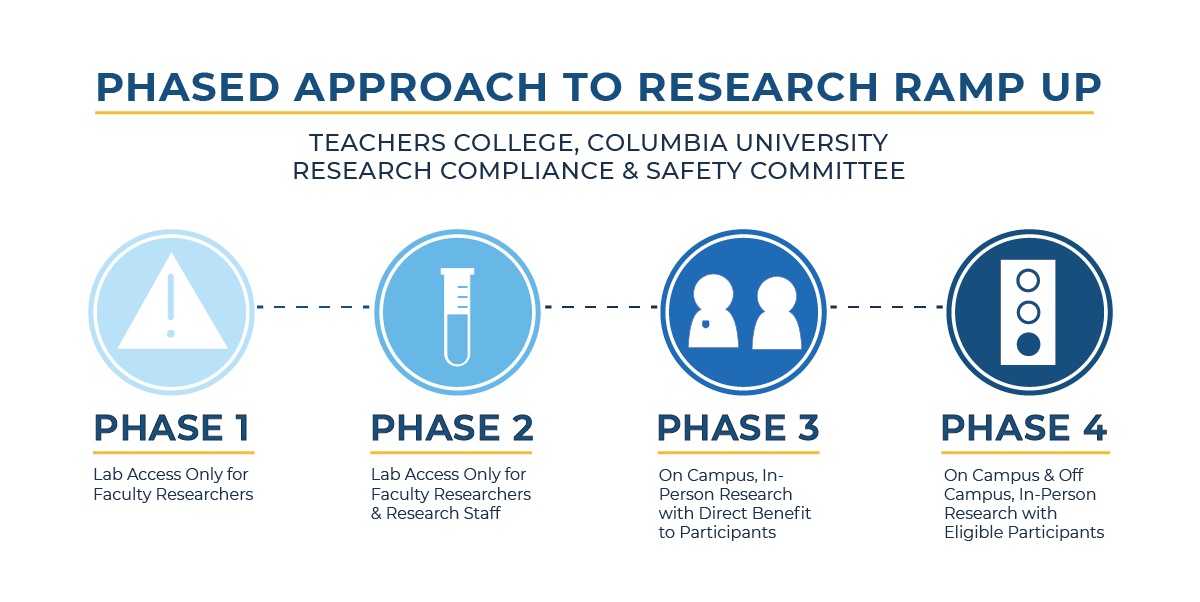
Phase 1 – Primary Researchers (Research Lab Lead Investigator) can return to campus for lab access only. Requires:
- Completed two-part (online and physical walk through of lab space) Job Safety Assessment and Risk Analysis
- General Return to Campus Training (hosted by Environmental, Health, and Safety)
Phase 2 – Research Assistants & Staff (students, staff, visiting scholars, etc.), can return to campus for lab access only. Requires:
- Each research team member completes General Return to Campus Training (hosted by Environmental, Health, and Safety)
- Coordination with Research Lab Lead Investigator

Phase 3 – With caution, researchers may restart on-campus in-person data collection for studies with direct benefit to participants. Requires:
- Completed two-part (online and physical walk through of lab space) Job Safety Assessment
- General Return to Campus Training (hosted by Environmental Health and Safety)
- Completed Research-Specific Training Modules (hosted on Return to Campus Research Training Hub website)
- Designated Research Safety Monitor Agreement for Lead Investigator, and if applicable, other research personnel
- Communicate with TC Institutional Review Board about in-person research intentions in an IRB modification to an existing IRB protocol or submitted as a new protocol for review (see https://www.tc.columbia.edu/institutional-review-board/ for more information). Researchers may not begin recruitment or research until they receive a final IRB approval letter. IRB protocols will be reviewed on a case-by-case basis.

Phase 4 – Three-part considerations (in-person research intentions must be reported to the IRB as a modification to an existing IRB protocol or submitted as a new protocol for review (see https://www.tc.columbia.edu/institutional-review-board/ for more information).
- International Studies where quarantine restrictions have already been lifted and local governmental regulations permit in-person contact.
- External Sites (especially those within hospitals or institutions who implement high safety and health standards).
- On-Campus with typically developing adults initially, and eventually vulnerable populations, who may be at higher risk.
- Requires:
- Completed two-part (online and physical walk through of lab space) Job Safety Assessment
- General Return to Campus Training (hosted by Environmental, Health, and Safety)
- Completed Research-Specific Training Modules (hosted on Return to Campus Research Training Hub website)
- Designated Research Safety Monitor Agreement for Lead Investigator, and if applicable, other research personnel.
- Communicate with TC Institutional Review Board with in-person research intentions as an IRB modification to an existing IRB protocol or submitted as a new protocol for review. Researchers may not begin recruitment or research until they receive a final IRB approval letter. IRB protocols will be reviewed on a case-by-case basis.