Risk Assessment
Risk Assessment in Research
There is always a level of risk involved in any research activity. The Institutional Review Board (“IRB”) is an administrative committee of Teachers College (TC) whose purpose is to ensure that the rights and welfare of human subjects of research conducted at or sponsored by TC regardless of the source of funding are protected pursuant to federal regulations (45 CFR part 46 and 21 CFR part 56). Examples of human subjects’ research include surveys, observations of behavior, experiments involving human responses, individual interviews, focus group sessions, and collection of data from existing records.
The IRB is required to assess the level of risk involved in a research study when making a determination for review requirements. Some items factoring into the IRB’s risk assessment include possible risk to a subject’s psychological wellbeing, or the risk to a subject if their confidentiality is compromised. The more risk involved in a study, the higher the level of review and the more human subject protections required by the IRB.
You can read more about the different levels of risk, and the associated review categories for each, on the IRB visit:
Review Categories | Institutional Review Board
All participants in research have the right to expect protection from physical, psychological, social, legal and economic harm at all times during study activities. Any participation in a research study is completely voluntary. An individual is free to decline to participate in a study for any reason. Even after they sign the consent form, they can still stop the study.
During times of significant risks, uncertainty, amidst a pandemic, or state of unrest within a region, researchers must take extra precautions to assess risk and ensure that they and others are safe. A researcher must stop a research study if they do not believe they can keep themselves or others safe. The research participant may also stop participating at any time or refuse to answer any individual questions.
If a researcher would like support with making such a decision, they can reach out to TC IRB (IRB@tc.edu).
Certain research may also present reputational, legal and / or economic risks to TC. As part of the ethical approval process for research involving human participants, researchers are required to identify potential risks associated with their research and the action they will take to mitigate risk. Depending on the risk level, researchers may be asked to submit their risk assessment to the IRB for review. The risk assessment process is a careful examination of what could cause harm, who/what could be harmed and how. It will help you to determine what risk control measures are needed and whether you are doing enough. For a guide on how to submit an IRB protocol, click here.
Here are some points for researchers to consider during the risk assessment process
Researcher Risk Assessment Checklist
- For faculty research leads of on-campus labs or spaces, confirm your role as the Research Safety Monitor (RSM)
- When you come to campus, consider prioritizing work that can only be completed in the workplace and delay work that can be done remotely, such as analysis of existing data
- For off-campus research, gather information about your host site and what safety precautions they have in place
- Identify emergency personnel who are essential to the operation of your research and make sure that they know what to do in the event of suspended operations
- Ensure you, as a researcher, and your research staff are prepared to keep yourself and others safe from the risks associated with viral spread, uncertainty, social unrest, or rapidly changing events. For a guide on the roles in research see “Roles & Responsibilities,” click here
- Review your communication plan and remind research personnel of who is in the notification chain
- Create a list of all research personnel and their contact information and remind all personnel to update their personal contact information
- Develop a workplace hygiene policy. Designate who should clean what and how
- Identify priorities in case of restricted access. You should discuss with your staff how work should be handled if some personnel are unable to come to work
- Ensure remote access to files, data, servers, etc. by working closely with Teachers College Information Technology (TC IT)
- Check that all members of your research team who might need to work remotely have access to computers that are able to connect to research files and data sets, research literature, and meeting software (e.g., Zoom).
- Learn about technology you may utilize to create a successful remote work environment.
- Familiarize yourself with the College’s travel restrictions before planning any travel. Visit the Office of Risk Management for information about international travel
- Check the Research Compliance & Safety Committee FAQs section
- Check TC IRB’s FAQs section
- Contact the Office of Sponsored Programs (OSP) to learn what to do if you believe that you will not be able to meet a grant deadline or need special assistance to do so
- Visit relevant websites, such as Teachers College Preparedness, Columbia University Preparedness, TC IRB, TC IT, Environmental Health and Safety, Office of Risk Management, Office of Sponsored Programs, Office of Teacher Education, Office of Access & Services for Individuals with Disabilities, Office of International Students and Scholars, and Human Resources, or others to find out more about ways to protect yourself and others
Occupational Risk Categories for Exposure to COVID-19
The Occupational Safety and Health Administration (OSHA) has determined categories of risk for likely exposure to viruses during an outbreak for American workers. The determination of your risk level will depend on the specific activities that are carried out as part of your research work.
- The Job Safety Assessment (JSA) is an important part of the process for ramping up in-person research on campus within research labs or spaces. To find out more about the JSA, and/or to schedule one for your research lab, space, or group, please contact the Office of Environmental Health and Safety
- The full OSHA document about Workplace Risk, and the OSHA Guidance on Preparing Workplaces for COVID-19, can be found here: Guidance on Preparing Workplaces for COVID-19
OSHA has divided job tasks into four risk exposure levels, as shown below.
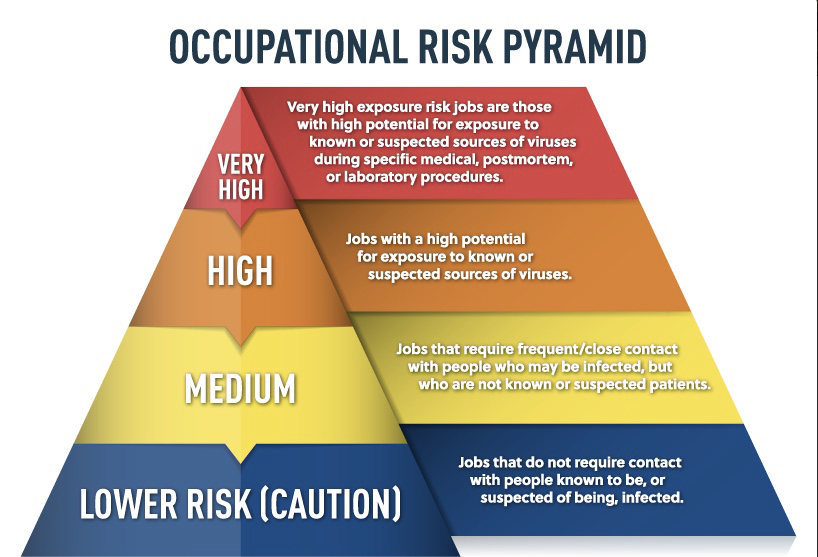
(Image Source: oshatrain.org)