FAQs
Teachers College’s (TC) Institutional Review Board (IRB) regularly receives frequently asked questions (FAQs) from researchers. Before contacting the IRB office, please check this list of questions to seek your answers. Please contact the IRB office if you have further questions or concerns, and have already checked other related parts of the TC IRB website (e.g. How to Submit).
IRB Overview
The Institutional Review Board (IRB) is an administrative committee of Teachers College (TC) whose purpose is to ensure that the rights and welfare of human subjects of research conducted at or sponsored by TC regardless of the source of funding are protected pursuant to federal regulations (45 CFR part 46 and 21 CFR part 56). Examples of human subjects’ research include: surveys, observations of behavior, experiments involving human responses, individual interviews, focus group sessions, and collection of data from existing records.
In taking on this responsibility the IRB ensures human subject research is conducted ethically and in compliance with the Belmont Report, applicable federal, state, local and institutional requirements by performing prospective and continuing review of the protocol, the informed consent process,and the procedures utilized to enroll subjects.
Research is defined as a systematic investigation—including research development, testing, and evaluation—to develop or contribute to generalizable knowledge. Investigations designed to develop or contribute to such scholarly knowledge are often made public beyond a single individual or internal program (i.e., publications, proceedings, or conference presentations). However, research that is never published is still research. Participants in research studies deserve protection regardless if the research is published or presented. A human subject is a living individual about whom a research investigator (whether a professional, faculty, or student) obtains data through intervention or interaction with the individual or from individually identifiable information.
A project requires IRB review if it includes both research and human subjects. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review.
Identifiable means the information contains one or more data elements that can be combined with other reasonably available information to identify an individual (e.g., Social Security number). Researchers should consult the IRB office if they are unsure about certain issues such as identifiable materials needing review or certain state laws regarding human subjects.
The term "publicly available" is intended to refer to records readily available to the broad public (e.g,. census data). A researcher should not assume information qualifies as "publicly available" merely because it has been posted on an electronic website and can be accessed without authorization.
In 1978, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research created The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The Belmont Report sets forth the basic ethical principles required for research involving human subjects. The Belmont Report encompasses three key principles: respect for persons (autonomy), beneficence, and justice.
To approve any proposed research to be conducted at Teachers College, the IRB shall determine that all the following requirements are satisfied:
- Risks to subjects are minimized: (i) by using procedures which are consistent with sound research design and which do not unnecessarily expose subjects to risk, and (ii) whenever appropriate, by using procedures already being performed on the subjects for diagnostic or treatment purposes.
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result. In evaluating risks and benefits, the IRB should consider only those risks and benefits that may result from the research. The IRB should not consider possible long-range effects of applying knowledge gained in the research (for example, the possible effects of the research on public policy) as among those research risks that fall within the purview of its responsibility.
- Selection of subjects is equitable. In making this assessment the IRB should take into account the purposes of the research, and the setting in which the research will be conducted. The IRB should be particularly cognizant of the special problems of research involving populations such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons. For research involving children and prisoners, the IRB ensures all special provisions detailed in 45 CFR 46.107
- Informed consent shall be sought from each prospective subject or the subject's legally authorized representative, in accordance with, and to the extent required by 45 CFR 46.116.
Training & Research Preparation
IRB protocols must be submitted before participant recruitment or research begins. Please review our Meetings & Deadlines page for information regarding the length of the review process. It is important that researchers allocate an adequate amount of time for the review process before scheduling to meet with participants.
Depending on the review category of your research, the review of a protocol will vary in length of time. A project requires IRB review if it includes both research and human subjects. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review.
Prior to beginning research, you will need to complete CITI Human Subject Protection Training. All Key Personnel are required to take human subjects protection training. For help with beginning your training, please visit our Training & Certification page.
Research may undergo one of three types of review:
- Exempt Review
- Expedited Review
- Full Board Review
The type of review depends on the risk level of the research and if the research falls into one of the expedited or exempt categories as defined by the federal regulations. Please see our Review Categories page for help determining the correct review type. The Institutional Review Board at Teachers College will make the final determination of a review category and whether a study requires review.
Below is a list of common research approaches:
- Analyses of existing data or biological specimens
- Cognitive and perceptual experiments
- Content analysis
- Descriptive, correlational, quasi-experimental, or experimental methods
- Epidemiological studies
- Evaluations of social or educational programs
- Interviews and focus groups
- Knowledge tests
- Medical chart reviews
- Observations
- Randomized controlled trials
- Surveys and questionnaires
Studies that are NOT human subjects research typically include:
- Internal studies, not made public, including data collections for internal departmental, school, or other institutional administrative purpose (e.g. office procedure evaluations, teaching evaluations, or customer service surveys).
- Information-gathering interviews where questions focus on items, products, procedures, or policies and are not about people, their perceptions, or thoughts regarding themselves. Information-gathering interview are typically not human subjects research (e.g. when individuals ask professionals product description questions, conduct office policy inquiries, or gain information from teacher-trainers about their upcoming workshop plans).
- Course-related activities designed specifically for educational or teaching purposes where data is collected from and about human subjects as part of a class exercise or assignment but is not intended for use outside of the classroom (e.g. instruction on research methods and techniques)
- Biography or oral history research involving a living individual that is not generalizable beyond that individual’s experiences. An oral history may include an individual's description of events earlier in their life. Interviews are designed to document events and lives that might otherwise be lost or forgotten.
- Case histories that are published and/or presented at national or regional meetings are often not considered research if the case is limited to a description of the clinical features and/or outcome of a single patient and do not contribute to generalizable knowledge. A case history is designed to record the past, an environment, or relevant details of a case and used as an example, analysis, or illustration of the event (e.g., a 38-year-old adult female complained of chronic left ankle pain which led to a new joint stress-relieving physical therapy method).
- Publicly available data do not require IRB review. (e.g., census data, labor statistics).
The IRB reviews each study is reviewed on a case-by-case basis. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review. If you have questions about your potential study email IRB@tc.edu or call 212-678-4105.
Studies that are human subjects research typically include:
- Studies involving human subjects to test or design devices, products, or materials developed through research for human use (e.g. testing if interactive virtual reality (VR) experiences using biometric information contribute to individual mindfulness and relaxation)
- Intervention or interaction with individuals to collect data for research purposes (e.g. evaluation of teaching methods and programs, internet surveys about sugar consumption, research involving risky behaviors or attitudes, and open-ended interviews with minors about family values in a foreign country that contribute to generalizable knowledge)
- Studies using private information that can be readily identified with individuals, even if the information was not collected specifically for the study in question
- Studies that use bodily materials such as cells, blood, urine, tissues, organs, hair, or nail clippings, even if these were not originally collected for research purposes.
- Studies that intend to produce generalizable knowledge about categories or classes of subjects from individually identifiable information
- Studies that involve analysis of existing individually-identifiable private information
- Studies that use human subjects to evaluate environmental alterations (i.e. weatherization options, travel patterns, or habitat modifications)
Research belonging to any of the above categories must comply with the Federal Regulations and the institution's policies for the protection of human subjects. Research is reviewed on a case-by-case basis. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review. Researchers should email IRB@tc.edu if they have questions or concerns about their study design and whether it should be IRB reviewed.
No, observational studies of public behavior (including television and public Internet chat rooms) do not involve human subjects as defined when there is no intervention or interaction with the subjects and the behavior is not private.
Observational studies may include data collected for non-research purposes that may not constitute as human subjects research if individual identities are not available (e.g., programmatic data such as service statistics, crime statistics, or election returns) and the data is not private.
Studies based on data that are individually identifiable but are also publicly available may not constitute human subjects research. The IRB reviews each study is reviewed on a case-by-case basis. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review. If you have questions about your potential study email IRB@tc.edu or call 212-678-4105.
Typically, minors are classified as individuals under the age of 18 years old, but there are some exceptions.
Children are persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted (per 45 CFR 46.402 and a similar definition in 21 CFR 50.3). Only individuals who are “children” under the federal regulations are covered by the additional protections described in 45 CFR 46, Subpart D and 21 CFR 50. Who qualifies as a “child” depends on local laws for consent.
Individual states and countries may have different laws governing the legal age of consent for treatment or procedures involved in the research. Researchers should understand the laws, standards, norms, and customs of international sites and provide relevant information to IRB administrators on the IRB application. Remember, informed consent is a process, not just a form.
Generally, the primary location where the research will occur is defined as the site or research site.
Researchers conducting studies outside of Teachers College must obtain site permission before conducting research at that site. Research conducted at TC typically includes two different types of external sites: schools (public or private) and organizations (or companies). Visit MyTC/Mentor IRB/Documentation for access to the site permission form templates. If researchers are recruiting participants from institutions other than Teachers College, they should include a site permission form (e.g., Site Permission Template) or a pending IRB approval from the institution(s) with their IRB protocol submission. Data collected in public locations (i.e., coffee shops or public parks) do not require a site permission form.
On an IRB application, researchers should describe the location, setting, and timing of data collection (e.g., face-to-face interview at a mutually convenient location, at the start of the semester). They should also include the state, city, school district, etc.
If any part of the research will occur within NYC Department of Education (DOE) schools, it is required that the researcher receive approval from Teachers College IRB prior to submitting their application to the Department of Education IRB (DOE IRB).
Roles & Responsibilities
The Principal Investigator (PI) is the primary individual responsible for the preparation, conduct, execution, and administration of a research grant, cooperative agreement, training or public service project, contract, or other research projects in compliance with applicable laws and regulations and institutional policy governing the conduct of research.
As the PI of record for a protocol, you are required to:
- Use current, up-to-date IRB approved documents
- Ensure all study staff and their CITI certifications are on record with the IRB
- Notify the IRB of any changes or modifications to your study procedures
- Alert the IRB of any adverse events
- Respond if the IRB communicates with you directly about any aspect of your protocol
Each PI must confirm:
- that they will comply with all regulatory compliance directives
- that all information submitted within the proposal is true, complete, and accurate to the best of their knowledge
- that any false, fictitious, or fraudulent statements or claims may subject the investigator(s) to criminal, civil, or administrative penalties
- that the investigator(s) agree to accept responsibility for the conduct of the project and to provide all required reports as applicable if a project is awarded as a result of the proposal
A principal investigator must be listed on the TC IRB protocol and CITI trained (please see Training & Certification for more information) within the last three years.
The PI must confirm that they understand their responsibility to abide by TC IRB's and the sponsor's policies, procedures and directives for the proper administration of research with human subjects. Failure to adhere to your responsibilities as a study PI can result in action by the IRB up to and including suspension of approval and cessation of the research.
Individuals who can be a PI include:
- All tenured and tenure-track Assistant, Associate, and Full Professors
- All persons holding appointments as a Research Assistant Professor, Research Associate Professor, Research Professor, Extension Specialists, and Clinical Professors
- All persons holding Adjunct, Visiting, Emeritus or other faculty positions at Teachers College*
- Post-Doctoral Fellows who have the approval of the supporting Department
- Research staff who have the approval of the supporting Department
- Graduate students may be designated as PIs with the approval of a tenured or tenure-track Assistant, Associate, or Full Professor.
*Adjunct Lecturers are considered to be non-tenured faculty. Non-tenured faculty usually have contract positions and hold ranks such as lecturer, instructor, or visiting assistant professor. To ensure compliance in human subject research, non-tenure and non-tenure track faculty members at Teachers College (TC) must obtain a sign letter of support from their Department Chair or authorized designee. For more guidance please refer to the Guidance for Supporting Non-Tenure Faculty in Human Subjects Research to learn more about obtaining support.
A Co-Investigator (Co-PI) is key personnel who have responsibilities similar to that of a PI on research projects. While the PI has ultimate responsibility for the conduct of a research project, the Co-PI is also obligated to ensure the project is conducted in compliance with applicable laws and regulations and institutional policy governing the conduct of sponsored research.
Each Co-PI must confirm:
- that they will comply with all regulatory compliance directives
- that all information submitted within the proposal is true, complete, and accurate to the best of their knowledge
- that any false, fictitious, or fraudulent statements or claims may subject the investigator(s) to criminal, civil, or administrative penalties
- that the investigator(s) agree to accept responsibility for the conduct of the project and to provide all required reports as applicable if a project is awarded as a result of the proposal
A Co-PI must be listed on the TC IRB protocol and CITI trained (please review our Training & Certification page for more information) within the last three years.
The Co-PI must confirm that they understand their responsibility to abide by TC IRB's and the sponsor's policies, procedures, and directives for the proper administration of research with human subjects. Failure to adhere to your responsibilities as a study Co-PI can result in action by the IRB up to and including suspension of approval and cessation of the research.
The role of a research coordinator (RC) can vary by project. However, typically the RC coordinates and administers research study-associated activities, assists in project planning, and ensures that the pre-established work scope, study protocol, and regulatory requirements are followed. As guided by the primary investigator (PI), the RC recruits and coordinates research subjects and serves as an administrative liaison for the project. The RC may oversee and coordinate the provision of research administration to direct the investigators or develop and maintain record-keeping systems.
A research coordinator must be listed on the TC IRB protocol and CITI trained (within the last three years; please see Training & Certification for more information). PIs should take caution when designating an individual with an RC role, as the RC can make administrative changes to a protocol in Mentor IRB.
To add an RC to your protocol, PIs must first designate the individual as a coordinator in their lab or center within the Mentor IRB system. Once this is complete, the RC must then be added to each relevant protocol via a minor modification.
For a step-by-step guide on assigning a research coordinator in Mentor IRB, please refer to the TC IRB Researcher Guide on How to Assign a Research Coordinator in Mentor IRB.
A research assistant (RA) is a researcher employed, often on a temporary contract, by a university, research lab, or a research institute for the purpose of assisting in academic research. Some RAs may volunteer on a project to gain research experience. RAs are not independent and are not directly responsible for the outcome of the research. Instead, RAs work under the guidance of a primary investigator or supervisor. RAs have view-only access through Mentor IRB.
All RAs must be listed on the TC IRB protocol and CITI trained (please see Training & Certification for more information) within the last three years.
Yes, students can be listed as a principal investigator (PI) of an IRB protocol with faculty sponsor support. Students listed as a PI are held to the same responsibilities as any other TC researcher. As the PI of record for a protocol, you are required to:
- Use current, up-to-date IRB approved documents
- Ensure all study staff and their CITI certifications are on record with the IRB
- Notify the IRB of any changes or modifications to your study procedures
- Alert the IRB of any adverse events
- Respond if the IRB communicates with you directly about any aspect of your protocol
A PI must be listed on the TC IRB protocol and CITI trained (please see Training & Certification for more information) within the last three years.
Failure to adhere to your responsibilities as a study PI can result in action by the IRB up to and including suspension of your approval and cessation of your research.
For more information on your Roles and Responsibilities as a Principal Investigator, please visit our Before You Begin page.
Adjunct Lecturers are considered to be non-tenured faculty. Non-tenured faculty usually have contract positions and hold ranks such as lecturer, instructor, or visiting assistant professor. To ensure compliance in human subject research, non-tenure and non-tenure track faculty members at Teachers College (TC) must obtain a sign letter of support from their Department Chair or authorized designee. For more guidance please refer to the Guidance for Supporting Non-Tenure Faculty in Human Subjects Research to learn more about obtaining support.
After securing Department support, students can create a TC IRB application through TC Mentor IRB. Within Mentor IRB, they are asked to select their faculty sponsor (last name first) from an auto-populated list. If your faculty sponsor’s name does not appear on the list, double-check the spelling of their name. If they still do not appear on the list, contact IRB@tc.edu.
After you select your faculty sponsor in Mentor and submit your protocol, they will receive this Faculty Sponsor Notification from Mentor. Your Faculty Sponsor will also be able to see your protocol. Please note, this notification should not be the first time your faculty sponsor sees your protocol.
A faculty sponsor can maintain a dual-role within a protocol (e.g. faculty sponsor and Co-PI).
Faculty sponsors hold an important role in guiding students who are planning or are in the process of submitting IRB applications. They are often actively involved in the student’s research, from protocol design to data analysis and report preparation. A faculty sponsor is required for all student IRB protocols. The faculty sponsor must approve the application prior to IRB review.
Administratively, faculty sponsors are selected through TC IRB Mentor (search via last name) from an auto-populated list. When you start a student protocol, you will be prompted to select your faculty sponsor before TC IRB will review your protocol.
After you select your faculty sponsor, they will receive this Faculty Sponsor Notification from Mentor. Your Faculty Sponsor will also be able to see your protocol. Please note, notification should not be the first time your faculty sponsor sees your protocol.
When a student first submits their IRB protocol, they must select a faculty member to accept the role as faculty sponsor in Mentor IRB. Please refer to the How to Accept: Faculty Sponsor Guide to follow the steps to accept the sponsorship and approve a student's protocol.
Yes, a new Faculty Sponsor Notification can be resent to the faculty sponsor by contacting the IRB@tc.edu.
A faculty sponsor may also directly acknowledge their role on a student protocol by logging in to the Mentor IRB system. To access the system, log in to the MyTC portal and click the Faculty or Employee Resources tab. On the right side, you will find a link to Mentor IRB. After logging in, click on the Student Protocols item in the column on the left. This protocol will be found on the resulting page.
If your faculty sponsor is having difficulty navigating TC Mentor IRB, you can email IRB@tc.edu and ask that a link to your protocol be sent to your faculty sponsor.
The Mentor IRB Alert System is a new feature designed to notify researchers of any pending actions related to their protocol submissions within the Mentor IRB system. This system aims to enhance communication between researchers and the IRB office by informing you about important deadlines, necessary revisions, and other critical information related to your research activities. If you receive a system-generated notice, it's important to take the required actions promptly, such as submitting a protocol withdrawal if the submission is no longer applicable, making the necessary revisions requested by the IRB reviewer, or contacting IRB@tc.edu for further guidance on how to proceed.
A study team needs to document IRB oversight of an external collaborator when:
- The project involves non-exempt research (i.e., a project is reviewed by the full board or expedited review process), and
- The external collaborator is engaged in the conduct of human subjects research (e.g., obtaining informed consent from subjects, interacting or intervening with subjects as part of the research, obtaining or analyzing personally identifiable subject data).
Notes:
- Formal IRB oversight for external collaborators involved in exempt research is generally not required. However, each institution is responsible for reviewing the involvement of their affiliated researchers in exempt projects to ensure compliance with institutional policies and ethical standards. When external collaborators are involved, institutions may choose to document roles and responsibilities through agreements or other mechanisms to maintain clarity and accountability.
- Collaborators are not considered "engaged in research" if they do not interact with subjects or the identified data, analyze de-identified data only, or assist with recruitment only. For more information," see the OHRP Guidance on Engagement of Institutions in Human Subjects Research (link is external).
New Protocols
Examine your study with the following questions in mind:
- Is the rationale for the study clearly stated and is the rationale scientifically sound?
- Are the aims and corresponding hypothesis clearly stated and is the primary outcome (and secondary outcomes, as appropriate) clearly defined?
- Is the question or hypothesis being tested providing important knowledge to the field and is the design of the study appropriate for the questions that are posed?
- Are statistical considerations, including sample size and justification, estimated accrual and duration, and statistical analysis clearly described and adequate to meet the study objectives?
The IRB reviews most study-related documents. You will need to assemble all study-related documents for IRB review prior to submission. The following documents should be included with your initial submission, as applicable:
- Documentation of training in the protection of human research subjects, completed within the last three (3) years (Training & Certification)
- New Study Application for Research Involving Human Subjects (i.e., IRB Application Template)
- If applicable, an Informed Consent Form Template (for adults competent to consent), a Parental Permission Form Template and/or an Assent Form for minors.
- Copies of all data collection tools, questionnaires, interview/survey forms, assessment materials, and descriptions of materials that subjects will encounter
- If applicable, advertisement(s), scripts, and postings for subject recruitment (e.g. IRB Recruitment Flyer Template)
- If applicable, a site permission form granting the researcher access to the site (e.g. School Permission Template and Site Permission Template)
- If applicable, a non-disclosure agreement, for a hired transcriptionist
- If applicable, a protocol giving a complete description of the proposed research in technical language.
- If applicable, a data-sharing agreement (Data Sharing Form Template) describing the relationship between institutions and researchers.
Each protocol is individually reviewed as it was originally submitted. Researchers should take care to submit final documents. Please do not submit rough draft documents, or documents with editor marks. For explicit details on what reviewers look for in an IRB protocol please download TC Reviewer Questions.
For TC IRB, an IRB application is a form you complete detailing the study parameters (IRB Application Template) For example, the listing the study activities (interview, survey, etc.) and the time it will take to complete each activity. The application may also include details about your research questions and study design.
The IRB protocol are the procedures you agree to follow while conducting the study in the field. If you need to modify your IRB protocol you can visit our Modification page and upload a Modification Memo describing any changes. If you have deviated from your IRB protocol, you can visit our Protocol Deviation page for more information.
For a more detailed walkthrough on how to submit changes to a protocol after it has been approved, please visit our How to Submit a Modification, Continuing Review, Adverse Event, and Deviation.
YES, 10,000 times yes! Think of it this way, IRB reviewers read hundreds of IRB protocols per month. Anything you can do to support ease-of-read makes IRB reviewers’ lives easier and ultimately your life better. Proofreading can also reduce the number of revisions you will need to make after your protocol has been submitted. Remember, ease-of-read and clarity should be your top priority. IRB reviewers may get frustrated if they cannot find where you made the changes, if the files are not properly labeled, or if you say you finalized a document, however, when opened the document still has tracked changes on it.
Please see our Writing for an IRB Review webpage for more information.
Doctoral Student Researchers should prepare to submit a new IRB protocol post-proposal hearing even if they may have submitted a pilot study for IRB review before their proposal hearing.
General Timeline for Successful Research
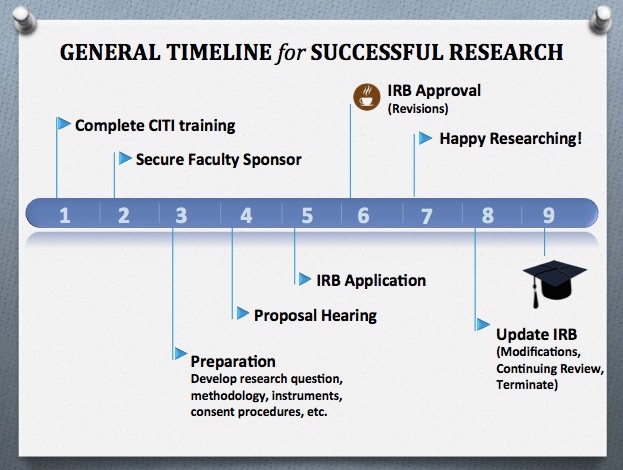
- Complete CITI Training
- Secure Faculty Sponsor
- Preparation - Develop research question, methodology, instruments, consent procedures, etc.
- Proposal Hearing
- IRB Application
- IRB Approval (Revisions)
- Happy Researching!
- Update IRB (Modifications, Continuing Review, Terminate)
Yes, doctoral students can conduct a pilot or pre-dissertation study. Depending on the study design, the doctoral student may or may not need IRB review and approval. For example, IRB submission is not required in instances when an individual meets with a group of friends to test the timing of the study measures and asks for their feedback. A pilot study or pre-dissertation study requires IRB if it meets the definition of “research with human subjects.”
Yes, all dissertation research must be reviewed by the IRB office. IRB reviewers will pay particular attention to when the doctoral student successfully defended their proposal hearing. The IRB office will not approve dissertation research until the proposal hearing has been successfully defended. Doctoral students can still conduct pre-dissertation research, pilot studies, and/or exploratory research before defending their proposal hearing.
A program evaluation offers researchers a systematic method for collecting, analyzing, and using information to answer questions about projects, policies and specific programs. The goal of program evaluations is to gauge the effectiveness and efficiency of a program. Program evaluations are typically reviewed at the exempt review category level but should still be submitted to TC IRB for review. The TC IRB will make the final determination of whether a study requires review. Researchers should email IRB@tc.edu if they have questions or concerns about their study design and whether it should be IRB reviewed.
Design experiments are pragmatic and theoretical in orientation even though they are conducted in a diverse range of settings. Experimental designs in schools may include:
- One-on-one teacher-experimenter and student designs where researchers train teachers in teaching practices and record the impact when compared to “business as usual,” instruction
- Classroom experiments with researchers and a collaborating teacher
- Preservice teacher development experiments to study the education of prospective teachers
- In-service teacher development studies
- School and school district restructuring experiments in which a research team collaborates with teachers, school administrators, and other stakeholders to support and institute organizational change in policy, procedures, or structures.
If you are conducting any research at a Department of Education (DOE) site, you must be reviewed and approved by TC IRB and then by DOE IRB. Here is a message from the DOE IRB regarding random controlled experiments:
“What is the IRB’s policy on randomized control trials? In developing a policy on the use of randomized control trials (RCTs), the DOE IRB considered both methodological challenges and ethical concerns. While RCTs have made contributions to the field of education, we believe RCTs are not the only way to examine causation, nor are they the one “scientific method/gold standard” with universal applicability. Randomization is considered the most powerful experimental design in clinical trials. However, a number of experts have questioned the applicability for behavioral research generally and specifically for education research. They have observed that highly complex organizations such as the educational system are a poor fit for the RCT model, which requires clear inclusion/exclusion criteria and interventions administered identically.”
For more information visit DOE IRB’s website.
The Data Security Plan includes best data security and privacy practices for researchers as determined by TC IRB and TC IT. The practices described in the data security plan should be implemented for all study activities and recruitment. A Data Sharing Agreement should be used when transferring data between institutions or researchers (e.g., a school shares a dataset with a Teachers College researcher). The Data Release Form should be used when requesting access to a participant’s existing data (e.g., asking college students to release a class assignment to the research team). An Information Sheet may be requested by the IRB when the research includes the use of a software, application, or device. An Information Sheet should include a description of the software/device, the type(s) of data collected and how the data will be transmitted, how long the software/device will be installed for and how to uninstall it, how to properly wear the device, and any risks that may be encountered with the use of the software/device. If a study includes the use of technology, the IRB may ask researchers to consult with TC IT and share the findings from this meeting in the IRB protocol. The goal of the TC IT consultation is to gauge the data security, privacy, and confidentiality of the technology when used for human subjects research purposes.
Reviews & Revisions
After the pre-review process, researchers will likely receive a request for revisions memo from TC IRB. The request for revisions memo will include details about adjusting the IRB protocol submission to adhere with federal regulations and TC IRB policies. For a guide on responding to revision requests, please view our Request for Revisions Memo Template.
Request for revisions are typically addressed in Mentor IRB in three ways (memo, tracked changes, and final protocol copy):
- Memo: Address each point above one-by-one in a memo format. Copy each numbered item into your memo and include your response.
- Please download our Request for Revisions Memo Template to get started.
- Tracked Changes: Submit all documents that you were asked to revise (e.g. consents, recruitment material, application, etc.) using tracked changes to clearly show where you made the appropriate changes within the document.
- Final Version: Include a clean copy of all the files in the final format. These forms should be formatted as final documents. If approved they will be stamped with your IRB protocol number for you to distribute to potential subjects. Please leave a margin of at least 1" at the bottom of any consent forms for the IRB stamp.
To replace an item in Mentor IRB that you were asked to revise with an updated one, click on the page icon (with the maroon lines) next to the document in question and select "replace." Do NOT delete the old document. All revisions should be uploaded to Mentor IRB. Please email IRB@tc.edu when you have addressed these issues as the reviewer will not receive an automatic notice.
Yes, you will want to itemize the requested revisions and respond to them individually. Please see our Request for Revisions Memo Template for a guide on responding to revisions. There may some rare cases when responding to all the requested revisions is impossible. In those rare circumstances, please email IRB@tc.edu or call 212-678-4105.
Remember, ease-of-read and clarity should be your top priority. IRB reviewers may get frustrated if they cannot find where you made the changes, if the files are not properly labeled, or if you say you finalized a document, however, when opened the document still has tracked changes on it.
Submit a minor modification on MyTC/Mentor IRB/My Protocols. In the modification memo (Modification Memo), list the new researcher's name, TC UNI, or email address. If the individual is not TC affiliated, include their email address. For a detailed walkthrough on how to submit a modification, please see How to Submit a Modification, Continuing Review, Adverse Event, and Deviation.
All research staff must be CITI trained within the last three years. For more information on CITI training visit our Training & Certification page.
Yes, TC requires all key personnel to have a current and complete Human Subjects Protection certification. Key Personnel is defined as the Principal Investigator (PI), research collaborators, research coordinators, research assistants, external researchers, research staff, administrators, and other investigators who are directly involved in conducting research with study participants or who are directly involved with handling private information related to study participants during a research project. Please direct the researcher to our Training & Certification page to get CITI certified.
Yes, just focus on ease-of-read and clarity. You can upload supplementary documents to match the requested changes. Please see our Modification Memo for a guide to submitting changes. For a detailed walkthrough on how to submit a modification, please review our Modification page and Common Types of IRB Modifications blog post.
A protocol modification is no longer considered minor when participant risks have changed or study activities diverge from the original protocol submission. One way to gauge study changes that may warrant a significant modification is to identify if the research questions have changed and if the study procedures also need to change to match the trajectory of the study. Please see our Modification page for more information.
Participant Recruitment & Permissions
To each participant, researchers shall provide:
- A statement that the study involves research, an explanation of the purposes of the research, the expected duration of the subject's participation, a description of the procedures to be followed and identification of any procedures which are experimental
- A description of any reasonably foreseeable risks or discomforts to the subject
- A description of any benefits to the subject or to others which may reasonably be expected from the research
- A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject
- A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained
- For research involving more than minimal risk, an explanation as to whether any compensation and/or medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained
- Any financial contributions made to the research team or College
- An explanation of who to contact for answers to pertinent questions about the research and research subjects' rights and whom to contact in the event of a research-related injury to the subject
- A statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled
When appropriate, one or more of the following elements of information shall also be provided to each subject:
- A statement that the treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) which are currently unforeseeable
- Anticipated circumstances under which the subject's participation may be terminated by the investigator without regard to the subject's consent
- Any additional costs to the subject that may result from participation in the research
- The consequences of a subject's decision to withdraw from the research and procedures for orderly termination of participation by the subject
- A statement that significant new findings developed during the research which may relate to the subject's willingness to continue participation will be provided to the subject
- The approximate number of subjects involved in the study.
IRB may approve a consent procedure that does not include or alters some or all of the elements of informed consent set forth in this section. Additionally, consent is not just limited to a form; it is a process that takes place at many steps during the research study. Visit our Submitting a New Protocol page for links to all TC IRB consent documents.
The Institutional Review Board at Teachers College will make the final determination of whether a study requires review and the elements within that review (e.g., if to include a consent form, parent permission, or assent form, etc.). If you have questions about your potential study email IRB@tc.edu or call 212-678-4105.
Recruitment involves attracting and selecting suitable candidates for a project. It can be conducted through newspapers, email, posters, brochures, word-of-mouth, by internet, radio or television announcements, or by soliciting volunteers in public spaces.
In some cases, personal and sensitive information is gathered about the individual during the screening process. A participant eligibility screening may occur over the phone, face-to-face or using a survey (either online or paper-based). The IRB should have an assurance that the information will be appropriately handled. A simple statement such as "confidentiality will be maintained" does not adequately inform the IRB of the procedures that will be used.
Examples of issues that are appropriate for IRB review:
- What happens to personal information collected over the phone when the caller ends the interview or simply hangs up?
- Is the data gathered by a marketing company? If so, are names, etc. sold to others?
- What are your steps for protecting personal identifiers (e.g., phone numbers and names)?
- Are names of non-eligibles maintained in case they would qualify for another study?
- What will you do in instances when an individual expresses interest (and qualifies to be) in a study, but later declines to participate?
- Are paper copies of records shredded or are readable copies put out as trash? The acceptability of the procedures depends on the sensitivity of the data gathered, including personal, medical and financial. The Institutional Review Board at Teachers College will make the final determination of whether a study requires review and the elements within that review (e.g., if to include a consent form, parent permission, or assent form, etc.). If you have questions about your potential study email IRB@tc.edu or call 212-678-4105.
No, you may not begin recruitment or research until you receive a final approval letter from TC IRB. Final approval letters will come directly from the IRB office via email. You can retrieve a PDF copy of this approval letter from Mentor IRB.
Please note if you are using a Consent Form, Parent Permission Form, or Assent Form it will bear an official IRB authorization stamp only after final approval. Copies of the official IRB authorized stamped consent form and supporting documents must be used for your research work.
Working with vulnerable populations and conducting research involving individuals with impaired decision-making ability will be reviewed at the Full Board level (unless otherwise determined by the IRB office).
Vulnerable populations are comprised of individuals who have a diminished capacity to understand the risks and benefits for participation in research and to autonomously provide informed consent; these populations may need extra safeguards and considerations. An individual's decisional impairment may result from a psychiatric, organic, developmental, or other disorder that affects cognitive or emotional functions, or may result from the effect of drugs or alcohol. The impairment may be temporary, permanent or fluctuate.
The Institutional Review Board at Teachers College will make the final determination of whether a study requires review and the elements within that review (e.g., if to include a consent form, parent permission, or assent form, etc.). If you have questions about your potential study email IRB@tc.edu or call 212-678-4105.
Asking your own students (or the parents of your students) to participate in your research is intrinsically coercive. Parents and students will always feel compelled to participate, in spite of your intentions and assurances, or they may perceive some intangible benefit to participation that does not exist.
As part of ensuring that students and parents will not feel compelled to participate, teachers working with their own students should not review the data until the end of the grading period, once final grades have been submitted. Alternatively, teachers may ask a research assistant to recruit and collect data on their behalf. In this case, the teacher-researcher will not know which students participated in the study until after the semester is completed.
As part of your IRB application, you will be asked to submit a Guide for Working with Your own Students form. In addition, you should inform participants of the following: “I am your classroom teacher and a researcher. It is your choice to participate in this study. This study is voluntary. You do not have to participate. Your class grades or class standing will not be impacted if you choose (or not choose) to participate in this study. As the researcher on this project, will not reveal your personally identifiable information with any unauthorized individual outside of the research team.”
Consent, Risks, & Benefits
Standards of ethical research require that investigators fully inform human participants of the risks, benefits, and procedures they may experience by participating in a study. Respect for participants before, during, and after the study underlies effective informed consent of the subjects or their legally authorized representatives.
Informed consent means an individual voluntarily agrees to participate in a research study. It is a continual process and does not end with a single consent form.
Consent is “informed” ethically and legally if all study parts are fully explained to participants. These parts can include interviews, observations, surveys, and experiments such as randomized controlled trial studies. Investigators should ask potential participants if they read the informed consent and also offer ample opportunities to ask questions about the purposes, procedures, risks, and benefits regarding the research study. In the course of research, researchers are responsible for informing the participants that they can skip any question they choose not to answer or stop the study at any time without penalty.
In some instances, investigators can seek alternatives to obtaining the standard informed consent. For example:
- Not collecting physical signatures, i.e. online consent
- Waiver of written consent, i.e. oral consent
- Waiver of some or all of the informed consent process, i.e. when deception is used
There are groups of individuals who cannot volunteer to participate or for whom the risk of coercion is very high, including children and cognitively impaired individuals. Investigators should explore which options are most appropriate for their target population and research design.
It is important to note that while exempt research is not subject to federal regulations, the IRB strongly recommends that you provide participants with the basic elements of Informed Consent which include describing the research and procedures involved in your study, the risks, the benefits, how confidentiality will be protected, and a statement that participation is voluntary and participants may withdraw from the study at any time without penalty. Contact information for the PI and the TC IRB office should also be provided.
Yes, all studies with Informed Consent forms (Parent Permission or Assent forms) must contain an official IRB authorization stamp only after final approval. TC IRB is the only authorized department to issue a TC IRB authorization stamp. Copies of the official IRB authorized stamped consent form(s) must be used for your research work. Remember, informed consent is a process, not just a form.
Yes! Please review our following informed consent templates, assent templates, and parental permission templates:
If you are working with a population that does not speak or read English, you must translate the participant-related materials (e.g., consent, assent, recruitment flyer, etc.) into their preferred language. Please also review our examples of translated parental permission forms:
- English to Spanish Translated Site Permission Form
- English to Chinese Translated Parental Permission Form
- English to Portuguese Translated Parental Permission Form
Please remember that informed consent (assent and permission) is a continual process, not just a form.
Yes! Please see our examples of translated permission forms.
An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either:
- That the only record linking the subject and the research would be the consent document, and the principal risk would be potential harm resulting from a breach of confidentiality
OR
- That the research presents no more than minimal risk of harm to subjects and involves no procedures, for which written consent is normally required outside of the research context.
In cases in which the documentation requirement is waived, the IRB may require the investigator to provide subjects with a written statement regarding the research. The rationale for approving a partial or full waiver of informed consent is handled on a case-by-case basis. If a waiver is approved, the IRB will issue the primary researcher a "Waiver Granted" approval notification to document the waiver of consent.
You cannot guarantee confidentiality in a group setting. You should inform participants of the following: “Your identity will be known to other focus group participants and the researchers cannot guarantee that others in these groups will respect the confidentiality of the group. We will ask you to keep all comments made during the focus group confidential and not discuss what happened during the focus group outside the meeting.”
Data Collection, Maintenance, & Storage
A data-sharing agreement is a formal agreement that clearly defines what data are being shared, and how that data is to be used according to specific terms and conditions as outlined in the agreement.
Before conducting any research or sharing data, both parties should discuss anticipated data-sharing and data-use issues to document in the agreement. Such an agreement can help researchers build upon the work of others and maintain good faith in research collaborations. Shared data encourages accountability and transparency, allows for the reproducibility of experiments and results, and provides a foundation for scientific inquiry. A data-sharing agreement can also help manage expectations among the involved parties.
For an example template of a data-sharing agreement, please download our Data Sharing Form Template. In some cases, researchers may need to consult TC Office of the General Counsel (for legal advice) or Information Technology for consultation on how to best draft a data-sharing agreement. Please make sure to consult these sources before submitting to TC IRB.
Certain additional protections for students and parents are provided by federal regulations. The proposed use of student education records for research must comply with the requirements of the Family Educational and Rights Privacy Act (FERPA). For researchers conducting research at other institutions, please note that FERPA restricts researchers’ access to student records without written permission from parents of minors, or permission from students over the age of 18. While some exceptions to FERPA may be available in a particular case, investigators must contact each institution in which they will be conducting research and follow that institution’s FERPA policy, in addition to the requirements of the IRB.
For information on storing digital data, please see our Data Sharing, Requests, & Encryption. We also have the following data templates available for researchers:
Qualtrics enables you to create and take surveys. You can design your survey to gain feedback, conduct polls, or perform other research purposes. Results can be viewed in reports and can be downloaded within Qualtrics. You can also share surveys and results with collaborators.
TC's Information Technology offers tutorials on how to use Qualtrics. To access Qualtrics, visit MyTC scroll down and click TC Qualtrics (Survey Tool).
Research Match is a free and secure online tool created by academic institutions across the country with an aim to create a platform for research involving health and wellness. Research Match can be accessed at https://www.researchmatch.org/about/ Some TC studies may qualify for posting on Research Match.
Amazon Mechanical Turk (MTurk) is an Internet crowdsourcing marketplace and hub enabling individuals, researchers, and businesses to post and coordinate human labor or seek human subjects to perform research tasks, including completing a survey. Please review MTurk's Privacy Conditions, Participant Agreements, and FAQs to ensure that Amazon's confidentiality standards and capabilities meet your research expectations. Also, make sure that you understand the common phrases used in an MTurk setting including phrases such as “Mturk,” “Worker ID,” and “HIT.”
Recommendations for Using MTurk
On your consent form you may use “I agree,” or “I do not agree,” check boxes in place of signature lines since the study will occur online. For example, you can state, “Please indicate in the checkbox that you are at least 18 years old, [and any other relevant inclusion criteria] have read and understand this consent form, and you agree to participate in this online research study."
On the consent form, you may also consider including language like:
- “This study contains a number of attention checks to ensure that participants are paying attention and completing the tasks honestly and completely. As long as you read the instructions and complete the tasks, your HIT will be approved and you will be compensated. If you fail these checks, your HIT will be rejected and you will not be paid.”
- “Your participation is voluntary. You may stop participating at any time by closing the browser window or the program to withdraw from the study. Partial data will not be analyzed.”
If including a confidentiality statement on your consent form, it may be appropriate to state, “Your Mechanical Turk Worker ID will be used to distribute payment to you but will not be stored with the research data we collect from you. Please be aware that your MTurk Worker ID can potentially be linked to information about you on your Amazon public profile page, depending on the settings you have for your Amazon profile. We will not be accessing any personally identifying information about you that you may have put on your Amazon public profile page.”
You can further review academic resources like the following to gain more information about MTurk and to determine if it is a good fit for your research:
- Overview of MTurk
- MTurk Worker Demographics
- University of Massachusetts Amherst's MTurk Guidelines (please note, not all of these guidelines are upheld by TC IRB. TC IRB may hold different policies regarding use of MTurk for research with human subjects.)
- Concerns on Research conducted with MTurk Demographics: Paolacci, G., Chandler, J.,& Ipeirotis, P. G. (2010). Running experiments on Amazon Mechanical Turk. Judgment and Decision Making, 5, 411–419.
If the research involves human subjects who are not U.S. citizens or Department of Defense personnel and is conducted outside the United States, its territories, or possessions, the following should be considered.
- Are there adequate provisions to protect the privacy interests of participants?
- Are there adequate provisions for protecting the confidentiality of the data through coding, destruction of identifying information, limiting access to the data, or whatever methods that may be appropriate to the study?
- If the information obtained about subjects might interest law enforcement or other government agencies, has a certificate of confidentiality been obtained?
- Are the PI’s disclosures to subjects about confidentiality adequate?
For tips on protecting digital data in countries outside the U.S. please consult Teachers College Information Technology department. For more information on international research, please visit our International Research page.