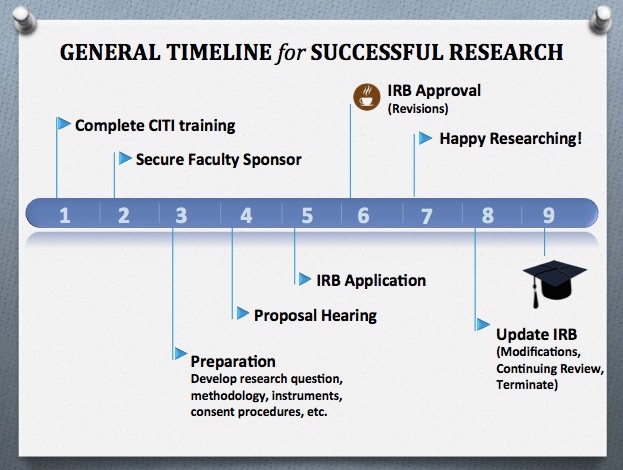
Before You Begin
When do I need to submit to TC IRB?
According to 45 CFR 46, a human subject is "a living individual about whom an investigator (whether professional or student) conducting research:
- Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
- Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens."
Are you conducting human subject research? Will your study contribute to generalizable (scholarly) knowledge? Have you developed research questions?
If so, reflect on the meaning of a systematic investigation, which is an activity involving a prospective plan that incorporates data collection, either quantitative or qualitative (or a combination of both), and data analysis to answer a research question. If your study includes both human subjects and systematic investigation, you will likely need to submit it to TC IRB. For further support in determining whether your study includes human subjects, please refer to the U.S. Department of Health and Human Services' Human Subject Regulations Decision Charts. A downloadable PDF version is also available here: Human Subject Regulations Decision Charts (pdf).
Please refer to our What is Human Subjects Research page for additional detailed examples of what studies are considered human subjects research and which are not.
What if I need a formal determination that my project does not involve human subjects research?
If you require an official determination regarding whether your project meets criteria for non-human subjects research (NHSR), please complete and submit the NHSR Determination application through Mentor IRB. For step-by-step instructions, refer to the TC IRB Walkthrough for Submitting a Research Determination Form in Mentor IRB guidance.
- A biospecimen is any biological material such as urine, blood, tissues cells, protein etc. that is obtained from humans for research purposes.
- Identifiable Private information includes Information that can be used or combined to ascertain the identity of a participant and their involvement in a research study. This information may include:
- Names
- Social Security Numbers
- Telephone Numbers
- Medical record numbers
- What is generalizable knowledge? The intent to engage in systematic investigation designed to contribute to generalizable knowledge meets the regulatory definition of research. Generalizable knowledge extends beyond the scope of the investigator, or an internal program and findings are shared publicly. This may include:
- The findings contribute to a theoretical framework or an established body of knowledge
- Publication, presentation at a conference, or other distribution of the results
- The results are intended to be replicated in other settings
Now that you have a better understanding of human subject research, generalizable knowledge, and systematic investigation, you will want to familiarize yourself with IRB regulations and review categories before beginning any IRB protocol. To begin, visit our Training & Certification page and review the information on how to get human subject research trained and certified. CITI certification is required before an IRB protocol can be approved.
IRB reviewers will determine, based on your actual submission, if your protocol is exempt, expedited, or full review. However, if you need additional support determining your IRB review category, please download the following documents:
- Is my protocol exempt from IRB review?
- Does my human subjects research fall under the expedited review category?
For further information on preparing your research study, please see the information below.
All Key Personnel involved with human research subjects must have a current and complete Human Subjects Protection Certification and should be familiar with federal regulations and TC IRB policies. Key personnel is defined as the Principal Investigator (PI), research collaborators, research coordinators, research assistants, external researchers, research staff, administrators, and other investigators who are directly involved in conducting research with study participants or who are directly involved with handling private information related to study participants during a research project. For more information, please see our Training & Certification page.
Please see our Review Categories page for a complete description of each category.
Exempt Research: Constitutes no more than minimal risk AND only involves human subjects in one or more of the following categories:
- Research conducted in established or commonly accepted educational settings, that specifically involves normal educational practices that are not likely to adversely impact students’ opportunity to learn or the assessment of educators. This includes most research on regular and special education instructional strategies, and research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
- Research that only includes interactions involving educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior (including visual or auditory recording).
- Research involving benign behavioral interventions in conjunction with the collection of information from an adult subject through verbal, written responses, including data entry or audiovisual recording if the adult subject prospectively agrees to the intervention.
- Secondary research for which consent is not required with private information or identifiable biospecimens collected for some other initial activity.
- Research and demonstration projects that are conducted or supported by a Federal department or agency, and that are designed to study, evaluate, improve, or otherwise examine public benefit or service programs.
- Taste and food quality evaluation and consumer acceptance studies, (i) if wholesome foods without additives are consumed or (ii) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture.
- Storage or maintenance for secondary research for which broad consent is required, including storage or maintenance of identifiable private information or identifiable specimens for potential secondary research use if an IRB conducts a limited IRB review and makes the determination.
- Secondary research for which broad consent is required, involving the use of identifiable private information or identifiable bio specimens for secondary research use
Note: Federal regulations indicate that certain research is exempt from review. However, under Teachers College's Policy for the Protection of Human Subjects, a research protocol proposing the use of human subjects must be submitted to the IRB to determine if it qualifies for exempt status. Exemptions do not apply to research conducted with pregnant women, prisoners, or vulnerable populations.
If you need additional support determining if your protocol is exempt, please review the following guide: Is my protocol exempt from IRB review?
Expedited Research: Constitutes no more than minimal risk AND only involves human subjects in one or more of the following categories:
- Clinical studies of drugs and medical devices only when condition (a) or (b) is met:
- (a) Research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. (Note: Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for expedited review.
- (b) Research on medical devices for which (i) an investigational device exemption application (21 CFR Part 812) is not required; or (ii) the medical device is cleared or approved for marketing and the medical device is being used in accordance with its cleared or approved labeling
- Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture.
- Prospective collection of biological specimens for research purposes by noninvasive means.
- Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving x-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. (Studies intended to evaluate the safety and effectiveness of the medical device are not generally eligible for expedited review, including studies of cleared medical devices for new indications.)
- Research involving materials (data, documents, records, or specimens) that have been collected or will be collected solely for non-research purposes (such as medical treatment or diagnosis). (Note: Some research in this category may be exempt from the HHS regulations for the protection of human subjects. 45 CFR 46.101(b)(4). This listing refers only to research that is not exempt.)
- Collection of data from voice, video, digital, or image recordings made for research purposes.
- Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. (Note: Some research in this category may be exempt from the HHS regulations for the protection of human subjects 45 CFR 46.101 (b)(2) and (b)(3). This listing refers only to research that is not exempt.)
- Continuing review of research previously approved by the convened IRB as follows:
- (a) Where (i) the research is permanently closed to the enrollment of new subjects; (ii) all subjects have completed all research-related interventions; and (iii) the research remains active only for long-term follow-up of subjects; or
- (b) Where no subjects have been enrolled and no additional risks have been identified; or
- (c) Where the remaining research activities are limited to data analysis.
- Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories 2 through 8 do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified.
If you need additional support determining if your protocol is expedited, please review the following: Does my human subjects research fall under the expedited review category?
Full Board: Studies that involve more than minimal risk require review at a fully convened IRB meeting. Studies that involve deception require full board review. The full board meets monthly. Meeting dates for the full board are posted in the Meetings and Deadlines page. Research submitted as a full review requires approval from a majority of IRB members.
Standard Review of Research: All IRB members have an opportunity to review or comment on any project approved by expedited or exempt review. If the Chairperson decides that a project should not be approved by expedited or exempt review, the project will be reviewed at the next convened full board meeting using the standard practices for new and continuation applications.
An IRB protocol may include materials for different audiences, including IRB reviewers, participants, and in some cases, parents or caregivers who will consent on behalf of the child participant. Researchers must distinguish between writing for a general audience, versus an academic or scientific audience. Discipline-specific language or jargon might not be appropriate for use with participants. Please visit our Writing for an IRB Review page for more information on writing for the correct audience.
Principal Investigators
The Principal Investigator (PI) is the primary individual responsible for the preparation, conduct, execution, and administration of a research grant, cooperative agreement, training or public service project, contract, or other research projects in compliance with applicable laws and regulations and institutional policy governing the conduct of research.
As the PI of record for a protocol, you are required to:
- Use current, up-to-date IRB approved documents
- Ensure all study staff and their CITI certifications are on record with the IRB
- Notify the IRB of any changes or modifications to your study procedures
- Alert the IRB of any adverse events
- Respond if the IRB communicates with you directly about any aspect of your protocol
Failure to adhere to your responsibilities as a study PI can result in action by the IRB up to and including suspension of your approval and cessation of your research.
Co-Investigators
A Co-Investigator (Co-PI) is key personnel who has responsibilities similar to that of a PI on research projects. While the PI has ultimate responsibility for the conduct of a research project, the Co-PI is also obligated to ensure the project is conducted in compliance with applicable laws and regulations and institutional policy governing the conduct of sponsored research.
Each PI and Co-PI must confirm (a) that they will comply with all regulatory compliance directives; (b) that all information submitted within the proposal is true, complete, and accurate to the best of their knowledge; (c) that any false, fictitious, or fraudulent statements or claims may subject the investigator(s) to criminal, civil, or administrative penalties; (d) and that the investigator(s) agree to accept responsibility for the conduct of the project and to provide all required reports as applicable if a project is awarded as a result of the proposal.
The PI and Co-PI confirm that they understand their responsibility to abide by the IRB's and sponsor's policies, procedures and directives for the proper administration of research with human subjects.
Who Can be a PI?
- All tenured and tenure-track Assistant, Associate, and Full Professors.
- All persons holding appointments as a Research Assistant Professor, Research Associate Professor, Research Professor, Extension Specialists, and Clinical Professors.
- All persons holding Adjunct, Visiting, Emeritus or other faculty positions at Teachers College.
- Post-Doctoral Fellows who have the approval of the supporting Department.
- Research Staff who have the approval of the supporting Department.
- Graduate students may be designated as PIs with the approval of a tenured or tenure-track Assistant, Associate, or Full Professor.
A study team needs to document IRB oversight of an external collaborator when:
- The project involves non-exempt research (i.e., a project is reviewed by the full board or expedited review process), and
- The external collaborator is engaged in the conduct of human subjects research (e.g., obtain informed consent from subjects, interact or intervene with subjects as part of the research, obtain or analyze personally identifiable subject data)
Notes:
- Formal IRB oversight for external collaborators involved in exempt research is generally not required. However, each institution is responsible for reviewing the involvement of their affiliated researchers in exempt projects to ensure compliance with institutional policies and ethical standards. When external collaborators are involved, institutions may choose to document roles and responsibilities through agreements or other mechanisms to maintain clarity and accountability.
- Collaborators are not considered "engaged in research" if they do not interact with subjects or the identified data, analyze de-identified data only, or assist with recruitment only. For more information, see the OHRP Guidance on Engagement of Institutions in Human Subjects Research(link is external).
If you are a student, you must secure a faculty sponsor to support you in your research efforts. Faculty sponsors hold an important role in guiding students who are planning or are in the process of submitting IRB applications for review. Faculty sponsors should provide recommendations about research design aimed at reducing the risk to human subjects while also serving as a point of contact for human subject concerns and questions. A faculty sponsor is required for all students conducting human subjects research.
After you save your protocol in TC's Mentor IRB, your faculty sponsor will automatically receive an email notice requesting they accept their faculty sponsorship role in Mentor. The faculty sponsor must approve the application prior to IRB submission on Mentor. TC IRB will not review a student's IRB protocol until the faculty sponsor has approved their role on the protocol.
GDPR & PIPL Compliance Resources
In support of our commitment to international research ethics and data protection standards, we have created resources to assist researchers in complying with the General Data Protection Regulation (GDPR) and the Personal Information Protection Law (PIPL). These resources include comprehensive guidance on obtaining consent, secure data handling, and cross-border data transfers. We aim to help researchers uphold participants' rights and ensure robust data privacy in studies involving individuals from the EU and China.
Available Resources:
GDPR
- TC IRB Researcher Guidance for EU General Data Protection Regulation (GDPR) Compliance
- TC IRB GDPR Consent Notice Template
PIPL
- TC IRB Researcher Guidance for China’s Personal Information Protection Law (PIPL) Compliance
- TC IRB PIPL Consent Notice Template
Translation Resources
Researchers interested in working with non-English speaking participants must include completed translation verification forms in their application. All recruitment and study materials must be transcribed by verified translators/interpreters. If research is to be conducted internationally, researchers must also submit proper documentation to be approved by the IRB, the Office of Risk Management, and the Office of Global Engagement.
Documents for Translations and Research Across Borders
Research Projects & Open Science
Open science practices promote transparency, collaboration, and accessibility in research. If your study involves sharing data openly or publicly, additional ethical considerations must be addressed to ensure participant privacy, informed consent, and regulatory compliance. This section guides researchers on how to incorporate open science principles into their IRB submissions, including consent form considerations, data de-identification practices, and data-sharing requirements.
Guidance Document
- TC IRB Researcher Guidance: Open Science and IRB Submissions (PDF)
This guide outlines key considerations for TC researchers planning to incorporate open science practices into their research, including properly addressing data-sharing in IRB submissions, consent form language, and best practices for protecting participant privacy.
Quick Tips for Submitting Open Science Projects
- De-identify your data before sharing to protect participant privacy.
- Use clear consent form language to inform participants about potential data sharing.
- Implement tiered data access levels (e.g., public, registered, controlled) based on data sensitivity.
- Engage with the IRB early if you plan to share existing data not originally intended for open dissemination.
- Consult institutional resources (e.g., TCIT, OGC) to ensure compliance with data security and legal standards.