Training & Certification
Researchers working with human subjects may not begin recruitment or research until they receive a final IRB approval letter. All research staff and faculty sponsors submitting an IRB protocol must have an updated CITI training certificate on Mentor IRB (awarded within the last three years). Please visit CITI Program to update your human subjects protections certification.
What is CITI?
CITI Program – Collaborative Institutional Training Initiative – provides high quality, peer-reviewed, web-based educational courses to train individual learners in research, ethics, regulatory oversight, responsible conduct of research, and research administration. CITI’s mission is to:
- Enhance the knowledge and professionalism of investigators, staff, and students conducting research in the United States and internationally
- Educate members, administrators, and leadership of ethics committees that review and oversee research
- Promote ethical research at organizations through the education of research administrators and organizational leadership
CITI training is required prior to IRB approval of:
- A new study on which Key Personnel are named
- An amendment application adding Key Personnel to a previously approved study
- A continuing review application for a study on which Key Personnel are named
Who Needs CITI Training?
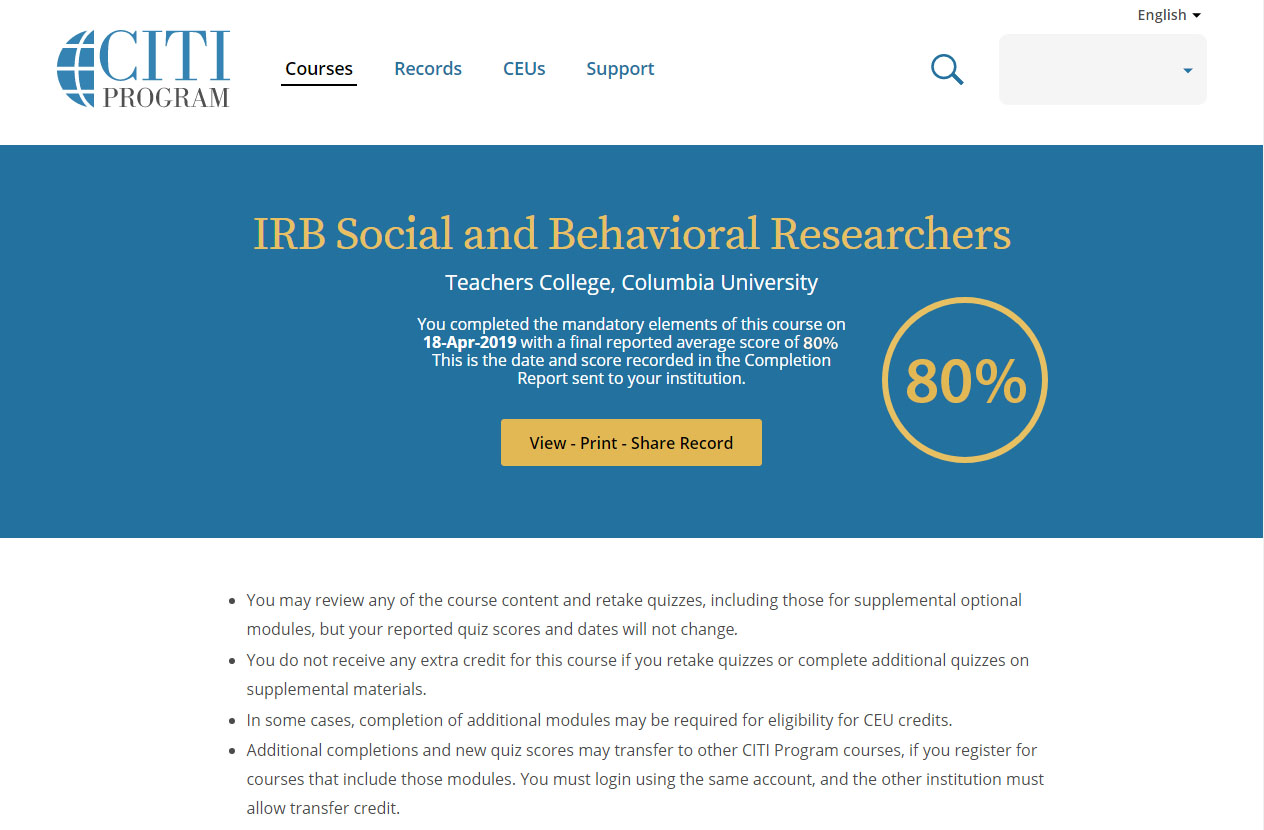
Before beginning research with human subjects, you will need to complete a basic training course through CITI. Please go to CITI program and register for the course using your TC affiliated email. All researchers with TC IRB must take the IRB Social and Behavioral Researchers Basic Course.
All TC researchers submitting to TC IRB must take the IRB Social and Behavioral Researchers Basic Course with CITI.
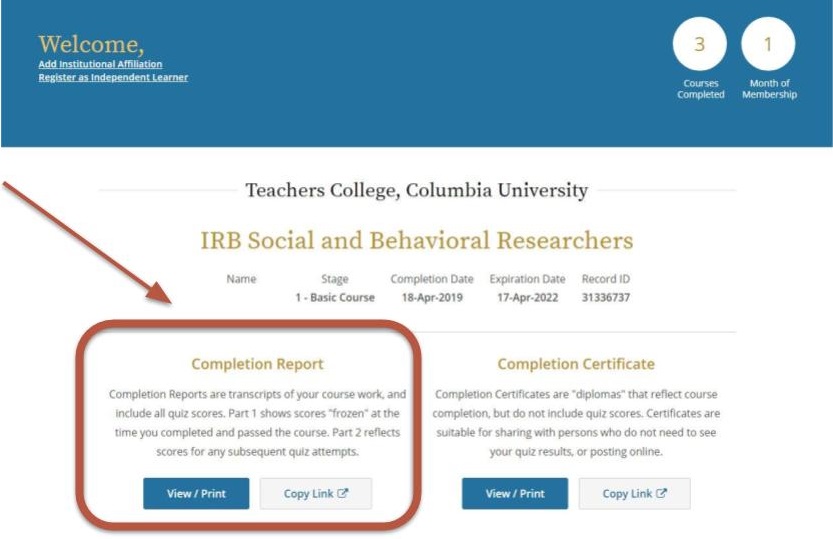
All Key Personnel involved with human research subjects must have a current and complete CITI training certificate (within the last three years) filed within Mentor IRB/PI Documentation. If your CITI training is expiring this year, please take the IRB Social and Behavioral Researchers refresher course, located in your CITI program profile. If the refresher course is not available, they can contact Dr. Myra Luna Lucero, Research Compliance Director at IRB@tc.edu or 212-678-4105 to request a formal expiration and access to the CITI refresher course. You can also direct them to TC IRB’s Certification webpage for more information. If they are no longer affiliated with the project, please submit a modification to remove them from the protocol.
Ethics & Safety Amid Uncertainty Training for In-Person Research
Key Personnel must complete the Ethics & Safety Amid Uncertainty Training course if they plan to use in-person methods at any point of their research, including during recruitment and data collection. Once training is concluded, please upload the completion certification to Mentor IRB.
What does Key Personnel in Research Mean?
Key Personnel is defined as the Principal Investigator (PI), research collaborators, research coordinators, research assistants, external researchers, research staff, administrators, and other investigators who are directly involved in conducting research with study participants or who are directly involved with handling private information related to study participants during a research project.