Human Research Protections: Updates
TC IRB is Hiring a Work-Study Research Compliance Assistant!
The institutional review board (IRB) has an open work-study position for a research compliance assistant. Research experience is a plus. This individual must have federal work-study through Spring 2023. Click here to apply!
Research Compliance Assistant Position
We are seeking a dynamic, self-directed research compliance assistant to support the creation of educational materials, facilitate daily office operations, and manage administrative tasks related to the Institutional Review Board (IRB) office.
The Research Compliance Assistant will:
- Follow established procedures and practices to ensure research involving human subjects complies with federal regulations, state laws, and College policies
- Support educational and informational material creation in response to College needs
- Assist with administration of IRB meetings and related duties
- Participate in training activities for students, staff, and faculty
- Have an opportunity to connect with research labs and centers in research compliance effort
- Under the supervision of IRB leadership, the individual will support office processes and policies to ensure smooth operation
- Work closely with the Coordinator and engage with filing and electronic processing of research related items
- May be required to attend IRB full board and subcommittee meetings
This position is remote through the summer and requires work-study. If you are interested, please apply here!

TC IRB and Graduate Writing Center Joint Session
April 18, 2022
Join the TC IRB's research writing intern, Jonthon Coulson, for joint session with the Graduate Writing Center for a workshop to explore digital resources and discuss research ethics while working across borders. The event will be April 22nd from 10:00-11:00 AM EST. Please find registration instructions below and bring your questions!
CITI Three Year Re-Certification Reminder
March 21, 2022
All research staff on an IRB protocol must have an updated CITI training certificate (within the last three years) filed within Mentor IRB/PI Documentation. If your CITI training is expiring this year, please take the IRB Social and Behavioral Researchers refresher course, located in your CITI program profile. If the refresher course is not available, contact Dr. Myra Luna Lucero, Research Compliance Director at IRB@tc.edu or 212-678-4105 to request a formal expiration and access to the CITI refresher course. Please TC IRB’s Certification webpage for more information.
Last Call for Applicants: TC IRB's Research Writing & Ethics Internship - Spring 2022
January 3rd, 2022
Are you a TC doctoral student interested in research writing and ethics? Apply to TC IRB's funded internship partnered with the Graduate Writing Center, Graduate Student Life & Development, and TC Next to gain research writing and ethics skills. Apply here!
Led by the Institutional Review Board (IRB) and in collaboration with the Graduate Writing Center, Graduate Student Life & Development, and TC Next, we are putting out a final call for Spring 2022 intern applicants!
TC IRB is looking for three doctoral students who will participate in a 10 week; 10 hours per week Research Writing & Ethics Internship for the Spring 2022 semester. Doctoral student interns who complete the internship will receive a stipend.
Doctoral student interns who participate in this program will:
- Accomplish challenging, but realistic tasks.
- Develop professional competencies for career success.
- Use the knowledge gained for specific research, writing, and ethics skills.
- Collaborate in a team environment and learn about campus research structures.
- Gain an opportunity to develop specific and measurable goals.
- Network with departments and colleagues including the Graduate Student Life &
- Development, TC Next, and the Graduate Writing Center.
If you are interested, please apply here! Deadline for submission is this Friday, January 7, 2022.

TC IRB's Research Writing & Ethics Internship - Spring 2022
December 6, 2021
Are you a TC doctoral student interested in research writing and ethics? Apply to TC IRB's funded internship partnered with the Graduate Writing Center, Graduate Student Life & Development, and TC Next to gain research writing and ethics skills. Apply here!
Led by the Institutional Review Board (IRB) and in collaboration with the Graduate Writing Center, Graduate Student Life & Development, and TC Next, we are proud to introduce the Research Writing & Ethics Internship!
TC IRB is looking to for three doctoral students who will participate in a 10 week; 10 hours per week Research Writing & Ethics Internship for the Spring 2022 semester. Doctoral student interns who complete the internship will receive a stipend.
Doctoral student interns who participate in this program will:
- Accomplish challenging, but realistic tasks.
- Develop professional competencies for career success.
- Use the knowledge gained for specific research, writing, and ethics skills.
- Collaborate in a team environment and learn about campus research structures.
- Gain an opportunity to develop specific and measurable goals.
- Network with departments and colleagues including the Graduate Student Life &
- Development, TC Next, and the Graduate Writing Center.
If you are interested, please apply here! Deadline for submission is Friday, January 7, 2022.

In-person research is back!
December 2, 2021
Dear TC Research Community:
The TC Institutional Review Board (TC IRB) office, in consultation with the Research Compliance & Safety Committee (RCSC), has moved to Phase 4 for in-person data collection (resumption of in-person research on and off-campus). Visit this link for a list of contacts participating on the RCSC.
In an effort to support researchers who plan to pursue in-person research, the RCSC and TC IRB office have developed the “Preparing Researchers for In-Person Engagement” Information Packet and accompanying IRB & In-Person Research website. The files are accessible to the entire TC Research Community, and are intended to be downloaded and adapted to suit the needs of each researcher. Individuals wishing to access the information packet must be logged in to myTC.
For COVID-19 research-related questions, please review Ethics Amid Uncertainty FAQs, or “Adapting Research in an Emergency” before contacting the IRB office ( IRB@tc.edu). If you have questions about whether proposed safety/hygiene standards are compliant with the Americans with Disabilities (ADA) Act, contact OASID via email (oasid@tc.edu).
Best wishes —
Professor Karen Froud, Ph.D., IRB Chair![]()
Dr. Myra Luna-Lucero, Ed.D., Research Compliance Director
Demystifying the IRB Seminar Session
November 29, 2021
In collaboration with the Graduate Student Life and Development, the TC IRB's Public Speaking intern Clara Haneul Yoon will host a session of Demystifying the IRB: IRB Trivia Extravanganza Seminar this December! The hour-long session will be a Virtual Trivia Extravaganza and will test your knowledge of the IRB and its process and you may also win a Starbucks gift card! The winner will also be spotlighted in our weekly GSLD newsletter. Please join us: https://bit.ly/IRB_Trivia
Session 3: Wednesday, December 8th, 3:00-4:00 PM EST
To register for the series please refer to the image below. To request disability-related accommodations please contact OASID at oasid@tc.edu.
TC NEXT Advising Sessions
November 19, 2021
Join a virtual session hosted by the TC IRB's Outreach & Career Development intern Zahra Ladhani, open to all doctoral students! Come together to discuss the process of writing your dissertation proposal and learn how your colleagues prepared for the IRB process. Please register below:
- Biobehavioral Sciences - Dec. 1 - 1:30 PM
- International & Transcultural Studies - Dec. 1 -4:00 PM
- Counseling & Clinical Psychology - Dec. 2 - 12:30 PM
- Math, Science & Technology - Dec. 2 - 4:00 PM
- Human Development - Dec. 3 - 11:00 AM
- Health & Behavior Studies - Dec. 3 - 1:00 PM
To request disability-related accommodations please contact OASID at oasid@tc.edu.

TC NEXT Advising Sessions
November 9, 2021
In collaboration with the TC NEXT, the TC IRB's Outreach & Career Development intern Zahra Ladhani will host advising sessions to support students interested in developing research. If you need help exploring ethical research feel free to check out TC IRB FAQs or book a session for one on one advisement.
Doctoral students who are in the process of exploring their research questions that may involve human subjects are invited to sign-up for a one-on-one peer support session through TC NEXT where we will:
- De-mystify the IRB process and how the IRB supports you in your research goal;
- Explore the resources available at TC IRB and TC NEXT for Doctoral students;
- Ultimately, how will the research they engage in support their career goals
To book an advising appointment please click here.
Demystifying the IRB Seminar Sessions
November 9, 2021
In collaboration with the Graduate Student Life and Development, the TC IRB's Public Speaking intern Clara Haneul Yoon will host two sessions of Demystifying the IRB seminar this November! The hour-long session covers the importance of the IRB for research, protocol submission tips, and the IRB criteria for review and approval. Please join us:
Session 1: Wednesday, November 10th 2:00-3:00 PM EST.
Session 2: Wednesday, November 17th 3:30-4:30 PM EST.
To register for the series please refer to the image below. To request disability-related accommodations please contact OASID at oasid@tc.edu.
Writing for the IRB - A Seminar Series
November 8, 2021
In collaboration with the Graduate Writing Center, the TC IRB's Research Writing Writing intern Stephanie Sheehan-Braine will host a two part seminar series starting this November! The series will help prepare and support researchers in writing for the IRB process. This two part series will include a guide for writing for the IRB as well as a roundtable discussion and session for feedback. In between each session, students will be encouraged to submit their IRB protocol for review by November 28th.
Part 1: A Guide to Writing for the IRB - November 19th 1:00-2:00 PM EST.
Part 2: Roundtable Discussion of IRB Feedback - December 3rd 1:00-2:00 PM EST.
To register for the series please follow the link here: https://bit.ly/2ZYldTx. To request disability-related accommodations please contact OASID at oasid@tc.edu.

ATTENTION DEPARTMENT, RESEARCH LAB, OR CENTER LEADS
July 21, 2021
This update content contains information for department, research lab, or center leads who wish to resume in-person activities with human subjects on- or off-campus in the Fall 2021 semester.
You can disregard this content if you:
- Are not a department, research lab, or center lead, or
- Do not plan to engage research participants in-person
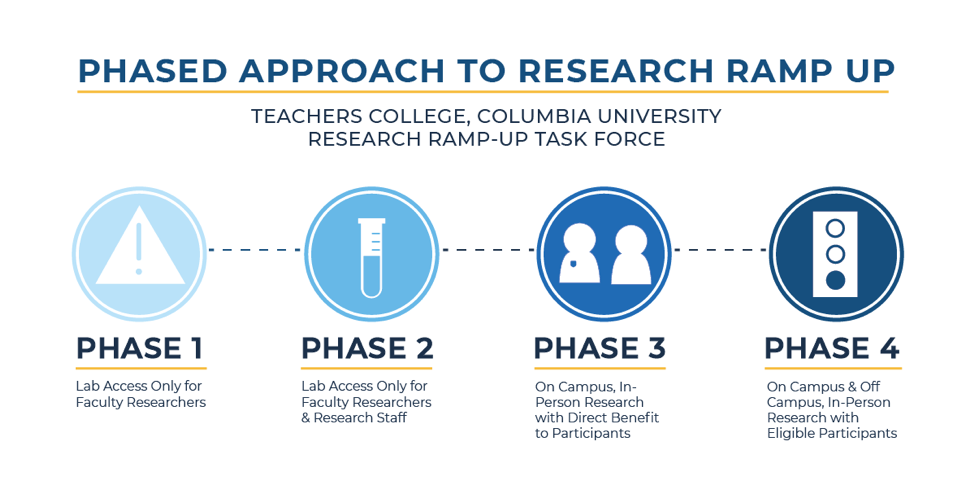
The TC Institutional Review Board (TC IRB) office, in consultation with the Research Compliance & Safety Committee (RCSC), has moved to Phase 3 for in-person data collection. Visit this link for a list of who is on the RCSC.
For COVID-19 research-related questions, please review Ethics Amid Uncertainty FAQs, TC’s Ethics Amid Uncertainty: Moving to Phase 3 of the Research Ramp-Up, or "Adapting Research in an Emergency" before contacting the IRB office (IRB@tc.edu).
Please see the attached "Gradual Return to In-Person Human Subjects Research Guide" and the Teachers College Fall 2021 Visitor Guidelines for more specific details.
Due to ongoing COVID-19 precautions, any study that can be conducted online or remotely should move forward with online procedures, and forgo in-person engagement.
Following “return to in-person research” procedures does not automatically mean that you can engage human research participant. Remember, recruitment or research cannot begin until you receive a final IRB approval letter. IRB protocols will be reviewed on a case-by-case basis. At this time, all proposed in-person research with human subjects will be reviewed by the IRB Full Board, regardless of its review category.
If you have questions, email IRB@tc.edu or set up a meeting using our TC IRB virtual office hours in which students, faculty, and staff can meet with an IRB administrator. Researchers can schedule a meeting in advance using our online scheduler. Virtual office hours are typically:
Tuesdays 10 AM to 2 PM
Wednesdays 11:30 AM to 2 PM
Thursdays 1 PM to 4 PM
Dates and times are subject to change.
Best wishes —
Karen Froud, Ph.D., IRB Chair![]()
Myra Luna-Lucero, Ed.D., Director of Research Compliance
LAUNCHED: Ethics & Safety Amid Uncertainty Research-Related Training Modules
December 7, 2020
Dear Teachers College Research Community —
We are writing as Co-Chairs of the Research Compliance & Safety Committee to announce the launch of the Ethics & Safety Amid Uncertainty Research-Related Training Modules. The training modules are one part of the multi-step process for returning to in-person research engagement, and they are now available through Canvas (UNI login required).
If you are a researcher who plans to resume in-person data collection, you are required to complete these training modules. In no case should in-person human subjects research begin without express approval from Teachers College (TC) Institutional Review Board (IRB).
The Ethics & Safety Amid Uncertainty training module requirement is in addition to the “TC COVID-19 Health and Safety” training required for all personnel.
The average completion time for the Ethics & Safety Amid Uncertainty training modules is approximately 2 hours, depending on reading speed. The modules are self-paced and do not have to be completed all in one session. If you are unable to access any of the resources that are part of these training modules, please contact OASID via email.
We have designed these modules to provide a set of resources that you can call on as needed. You will have access to the content on Canvas even after your initial read-through of the modules.
We hope you find the Ethics & Safety Amid Uncertainty Research-Related Training modules to be useful as we take steps towards the cautious resumption of in-person research activities.
A few reminders:
- Due to COVID-19 quarantine, all in-person study activities with human subjects are suspended. Following guidance from New York State and Teachers College Officials, the Institutional Review Board will announce when in-person research can resume and what steps to take at that time.
- We have a phased approach for in-person research, and we are currently at PHASE 2 of the research ramp-up process.
- We do not have a firm date of when in-person research can fully resume. We are monitoring the situation, consulting with experts, and reviewing IRB protocols on a case-by-case basis.
- Please consult the TC Preparedness Website for more information about the gradual resumption of campus life.
- In-person research remains paused until the Centers for Disease Control and Prevention lifts COVID-19 quarantine restrictions, campus administrators give approval, and TC IRB officially confirms that in-person research may begin. You can download walk-through guides for the COVID-19 modification process below:
We remain grateful for your flexibility and patience and wish you all continued health, safety, and productivity. Warm wishes for a restful holiday season!
Best wishes —
Karen Froud, Ph.D., IRB Chair![]()
Myra Luna-Lucero, Ed.D., Director of Research Compliance
On-Campus Research Moves to Phase 2
October 12, 2020
Dear Teachers College Research Community –
Now that the fall semester is thoroughly underway, we want to update you on progress towards resuming in-person human subjects research. As a reminder:
Due to COVID-19, in-person research data collection activities on the Teachers College campus, and at remote sites by Teachers College personnel, are suspended.
We are writing as Co-Chairs of the Research Compliance & Safety Committee, formerly the Research Ramp-Up Task Force. The goal of the committee is to harness content-specific expertise, initially to establish a plan for in-person research ramp-up at Teachers College with different phases that permit occupancy to be scaled depending on the scenario, personnel, and study site. Each phase should be adaptable as appropriate for the circumstances pertaining to specific research groups and risk levels.
The committee’s continuing mandate beyond the pandemic is to evaluate, establish and maintain aspects of regulatory compliance, risk management, and safety for researchers and research participants in the Teachers College community.

The Research Compliance & Safety Committee has served as a guide to the Institutional Review Board (IRB) on research-related matters and human subjects protection strategies in the time of COVID-19. We recently launched research-related information on TC’s Preparedness website including a four-phase process for returning to in-person research activities. We have been in Phase 1 for the initial parts of the semester while the College reopening procedures were established.
As of now, we are moving to Phase 2.
This means that Faculty Researchers and Research Staff, including students who work in research, may access on-campus labs and research facilities provided that all campus safety requirements are met.
Requirements for moving into Phase 2 are detailed here.
And include:
- Teachers College Environmental Health and Safety Training
- Daily completion of self-health check
- Recent negative COVID-19 test
Teachers College is implementing a 0% to 25% on-site employee model to achieve physical distancing in the workplace by maintaining remote operations for non-essential employees and office activities. Researchers returning to campus must adhere to this on-site campus model.
We are all aware that some research studies cannot sustain long-term in-person data collection suspensions. We anticipate being able to move to Phases 3-4 over the next several months and we are already working with individual research labs and centers to support this gradual transition.
Over the coming weeks, we will send out more information about requirements for resuming in-person data collection on-and-off campus—including the “Ethics Amid Uncertainty Research-Related Training Modules,” geared for researchers who will conduct in-person data collection. We will make an announcement when the link to these training modules is available on the Research Compliance & Safety website.
We aim to support researchers through the many hurdles in preparation for eventual in-person data collection. Changes or guidelines to in-person data collection will follow an iterative process. Each phase will require a feedback period as we refine and scale-up researcher guidelines. In other words, no changes will be made in haste and your patience through this process is essential. We are taking care to weigh decisions and assess risks for both researchers and research participants. In addition to our Committee, we are also consulting with campus staff and researchers familiar with safety and public health.
As a reminder, the procedures to become eligible to resume in-person data collection with human participants are threefold:
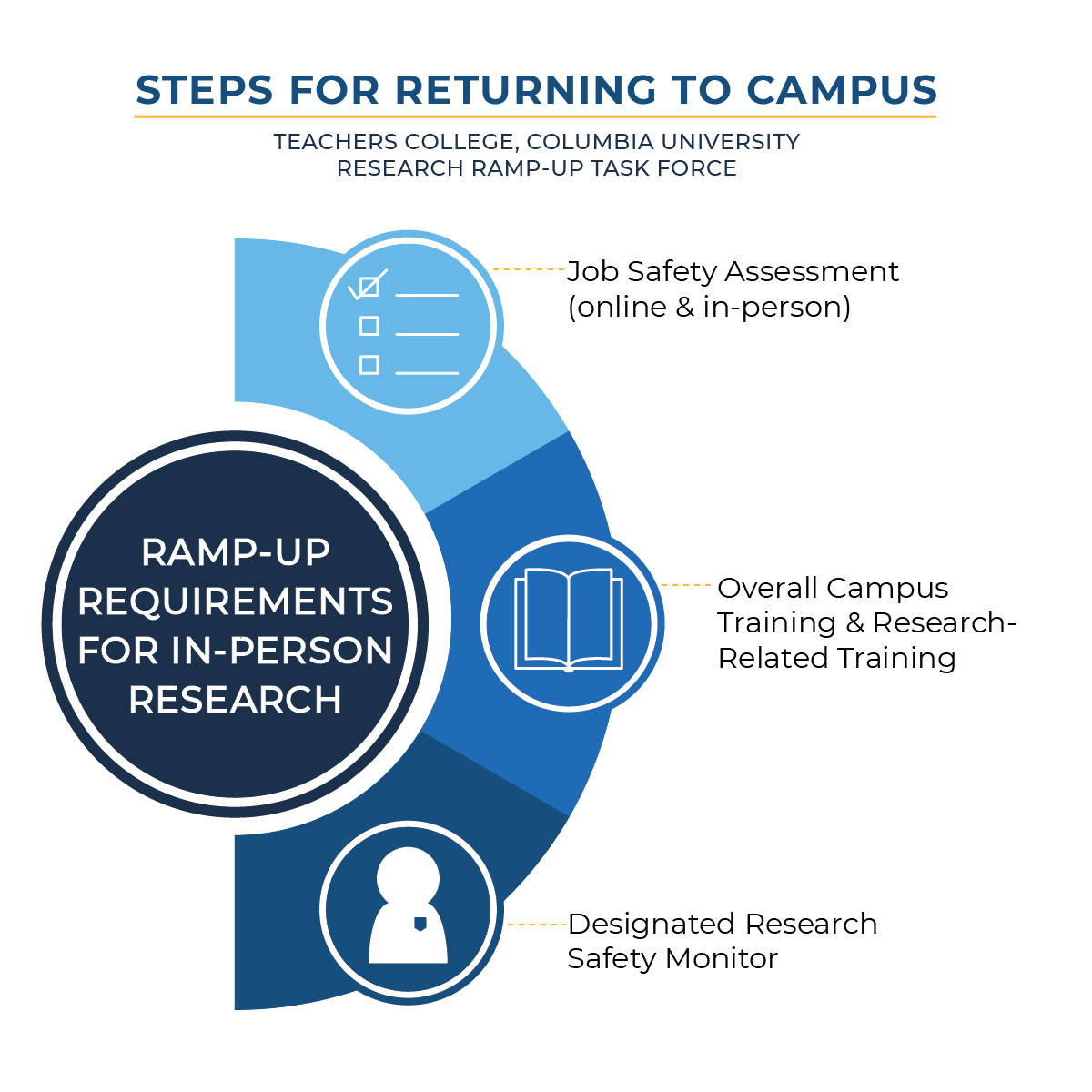
- Job Safety Assessments have been conducted with the Offices of Environmental Health and Safety and Facilities, and reports will soon go out to individual researchers detailing safety requirements determined as a result of those assessments. Please contact Patrick Mathelier, Director of Environmental Health and Safety (ehs@tc.columbia.edu) if you wish to schedule a Job Safety Assessment (JSA).
- The Teachers College Environmental Health and Safety Training is already available and is required in order to enter campus in any capacity. The “Ethics Amid Uncertainty Research-Related Training Modules,” is under development and will soon be available; this is required before researchers may resume in-person data collection activities.
- The role of a designated Research Safety Monitor (RSM) requires completion of the Job Safety Assessment and the “Ethics Amid Uncertainty Research-Related Training Modules.” The RSM will also be asked to complete an agreement to adhere to a hygiene and safety policy within their own research lab or center.
The Teachers College Institutional Review Board (TC IRB) will maintain oversight of research involving human subjects, and resuming in-person data collection will require IRB approval and the completion of these three steps.
We do understand that some research may be deemed essential, may carry direct benefits to participants, or may need to adhere to strict obligations or deadlines. If there are specific, extenuating circumstances that you believe constitute grounds for resuming in-person study activities ahead of the phased plan, please indicate this information in Mentor IRB for review and determination.
- You can do this by navigating to your protocol page in Mentor IRB. Click on the Modifications tab and select “Create New Modification.” Check the box next to "My Study Methods Cannot be Moved Online for COVID-19 and Study Cannot be Paused" and then, when the modification is created, click on the link to the "My Study Cannot be Moved Online" (links in Mentor IRB usually appear as dark red text). This step will take you to a short survey to provide more information as to why your study should include direct face-to-face interaction with a research participant. TC IRB will make the final determination on your modification submission.
- Please review the How to Submit COVID-19 Modification Studies Not Online walkthrough guide for studies that cannot moved online.
- We also have a walkthrough guide for instances when some of your research activities must remain in-person, yet others can be moved online. In these cases, please review the How to Submit COVID-19 Modifications for Hybrid Activities walkthrough guide.
Resuming in-person data collection with human participants will be dependent on continued monitoring of the health and safety of our community, and on approvals from TC IRB, the Offices of the Provost, and Environmental Health and Safety.
In the next few weeks, we will also post new researcher materials:
- A new External Site Permission form that includes safety and hygiene language
- Revised consent, permission, assent forms which include statements about:
- Person-to-person exposure is the most frequent route of transmission for infectious viruses and occurs via direct inhalation of respiratory droplets during close contact.
- Ways to mitigate risk (social distance, wear face covering).
- (Limited) Mandated Reporting: When required by law, information (including individually identifiable information) related to a research subject’s COVID-19 tests results may be reported to a public health authority.
We ask you all to continue to work remotely (online) for as long as possible. Minimizing the density of personnel on campus is crucial for everyone’s safety. Please do not engage in the return to research activities on campus unless this is crucial for your work. We will gladly help if you wish to have a discussion in order to make that determination. Please feel free to reach out by email with any comments, questions, or concerns.
If you would like to contribute your insights to the design of our procedures and policies, you can:
- Complete this confidential 15-minute survey.
- Volunteer for a 15-minute, semi-structured interview (email IRB@tc.edu, with a subject line of “Research Input for Research Ramp-Up”)
As ever, we are working hard to ensure everyone’s safety as we cautiously resume aspects of our research operations. We remain grateful for your flexibility and patience, and wish you all continued health, safety, and productivity.
Best wishes –

Karen Froud, Ph.D., IRB Chair
Myra Luna-Lucero, Ed.D., Director of Research Compliance

Research Ramp Up Update - July 1, 2020
July 1, 2020
Dear Teachers College Researchers,
On June 11, 2020 you received an email about “On-Campus Research Ramp Up Plans.” We are writing as Co-Chairs of the Teachers College Research Ramp-Up Task Force, to update you on the process towards ramping up research activities on campus. As a reminder:
Due to COVID-19, all research-related activities taking place on campus in-person at Teachers College are suspended.
You may be aware that Columbia University (CU) is now beginning to open up its research facilities to personnel. While we are building on many of the procedures that CU is using, we have our own concerns for our researchers – especially since most of our research involves human participants. Therefore, our process towards re-opening, and our timelines, differ from those being implemented by CU. In other words, we have intentions of ramping-up research-related activities on campus, but our plans are tailored to the needs of our researchers.
Below is a description of the Teachers College, Research Ramp-Up Plans:
Anyone returning to campus must engage in training modules and procedures that are being developed by the offices of the College President and Provost. The measures described here will be in addition to those requirements, and specific to the needs of each research group.
As a first step, we ask everyone who runs a research group to please complete this confidential 15-minute survey.
The survey is designed to help us understand who might need to return to campus, when they might need to return, and for what functions.
At the end of the survey, you can voluntarily share your email address if you would like someone to follow-up with you about any specific return-to-campus concerns.
We are preparing to roll out a THREE-STEP PROCESS for investigators who wish to resume selected research activities at Teachers College. This email serves to describe these steps and to inform you all of how you can engage with this process if on-campus activities are vital for your research.
Step One
All faculty lab directors who wish to resume on-campus research activities must first undertake a JOB SAFETY ASSESSMENT (JSA).
- To gather information for the JSA, faculty lab directors will be interviewed by representatives from our Environmental Health and Safety Office, the Office of Public Safety, and Facilities. The JSA will serve to identify the functions carried out by personnel in your research group, and any issues concerning the procedures you implement, the populations you work with, and the space that is occupied by your group. You can support the efforts of the JSA by engaging in initial discussions with your research lab about study activities, access to campus resources, and potential in-person research-related engagement.
- Based on the JSA, other requirements for your research group may be identified, such as reorganization of your research space, additional trainings needed to keep researchers (and eventual research participants) safe, or additional needs for personal protective equipment (PPE).
- We will email faculty lab directors to schedule JSAs starting next week. If you wish to schedule a JSA you can also contact us at IRB@tc.edu with a subject line of “Schedule a JSA Meeting.”
Step Two
After the JSA is completed, you must undertake ONLINE TRAINING MODULES that are specific to researchers on campus. The training modules are not yet finalized; however, they will be forthcoming. We will email researchers when the training modules are available.
- Depending on the JSA, you may be asked to take additional training modules (e.g., on procedures for protecting vulnerable populations, or on the application of specialized PPE).
Step Three
RESEARCH SAFETY MONITOR: Once you have completed the training modules, we will request that you complete a Research Lab Safety Plan and that you agree to act as the person responsible for overseeing the implementation of safety procedures in your research lab. In other words, you will serve as the Research Safety Monitor. You may be asked to complete specific Research Safety Monitor training modules.
- We are developing a RESEARCH SAFETY MONITOR AGREEMENT and a RESEARCH LAB SAFETY PLAN TEMPLATE to support this step, and these will be available to researchers as they move through the ramp-up process. We will email researchers when these documents are available.
- If you wish, you may nominate another person (NOT a student) from your research group to be a secondary Research Safety Monitor.
- Research Safety Monitors will be required to provide contact information so that they may be reached in the event of any emergency affecting their lab or research space, or in the event of any non-compliance with the Research Lab Safety Plan.
Please note that these procedures will not automatically mean that you can bring human participants onto campus. The campus remains closed to all visitors at this time.
If possible, we encourage researchers to conduct research activities online into the foreseeable future. Minimizing the density of personnel on campus is crucial for everyone’s safety. Please do not engage in the return to research activities on campus unless this is necessary for your work.
After researchers meet the three-steps listed above (Job Safety Assessment, Complete Required Training Modules, and Designate a Research Safety Monitor), they will also be expected to contact the Institutional Review Board (IRB) Office to update any approved protocols for a return to campus. For IRB questions, email IRB@tc.edu
We are currently pilot-testing the training modules, assessments, and agreements.
We are all aware that some research studies cannot sustain long-term in-person data collection suspensions. We are also aware researchers are under tight time constraints and are expected to complete multiple demanding tasks. With these considerations in mind, we are balancing training modules and requirements for a safe return to campus with reasonable time-based expectations. No plan or step will be perfect, but we are operating with care and consideration each step of the way and we appreciate your patience and understanding.
If you would like to contribute your insights to the design of our procedures and policies you can:
- Volunteer for a 15-minute semi-structured interview to voice your concerns (email IRB@tc.edu, with a subject line of “Research Lab Input for Research Ramp-Up”)
As ever, we are working hard to ensure everyone’s safety as we cautiously resume aspects of our operations. We remain grateful for your flexibility and patience, and we welcome your input.
Best wishes –
Dr. Karen Froud & Dr. Myra Luna Lucero
Update from the On-Campus Research Ramp Up Planning Committee
June 11, 2020
Dear Colleagues,
We hope this finds you all well and somehow hanging in! We are writing as Co-Chairs of the Teachers College Research Ramp-Up Task Force, to let you know about upcoming plans.
Due to COVID-19, all research-related activities taking place on campus in-person at Teachers College are still suspended.
However, we have been working hard to move forward responsibly and safely to ramp up inperson research activities on campus by the Fall term. Research activities may or may not include interactions with research participants, as we will need to assess risk based on various factors, including the nature of the activity and the vulnerability of the participant of the participant. We aim to respond to the needs of researchers while minimizing risks to participants and staff.
As recently communicated by Columbia University, the ramping-up of laboratory research requires satisfying two conditions:
- Official New York State permission to initiate research
- College approval to begin research as contingent on established policies and procedures in place so we can move forward with all the prudence required
Until this week, New York State was not permitting laboratory and clinical research activities to commence until New York City entered Phase Two of the Governor’s New York Forward Plan, which it has not yet achieved. This week, the Governor reclassified Higher Education Research as a Phase One activity. Since New York City entered into Phase One on Monday, June 8th, the first of the two conditions is now satisfied.
Determining when the second condition is satisfied is a matter for our own judgment, based on pilot testing, program evaluations, and consistent risk assessments. We cannot responsibly ramp up on-campus in-person research until we are confident that we have made all necessary arrangements for securing access to personal protective equipment, symptom-tracking procedures, facility readiness, training, and any other important safety measures. We are collecting survey data from research labs and centers and conducting semi-structured Zoom or telephone interviews to ensure that the measures we use for research ramp-up, and their implementation, are well designed. Of course, there will be limitations to any design and we will need to adapt procedures and policies as environmental or social changes occur. If you would like to contribute your insights to the design of our procedures and policies you can either:
- Complete any emailed electronic surveys geared at gauging the pulse of research
- Volunteer for a 15-minute semi-structured interview to voice your concerns (email IRB@tc.edu, with a subject line of “Research Lab Input for Research Ramp-Up”)
Ramping up in-person research activities will take time and we value your patience. Part of our plans include:
- Training preparations based on function and roles on campus:
- General health and safety trainings that meet Occupational Safety and Health Administration (OSHA) standards
- Training for working with participants in-person
- Training for researchers who work with vulnerable populations
- Training for researchers who work off-campus or conduct in-home visits
- Determining who needs to take on specific responsibilities;
- Deciding which labs and centers may open, and when;
- Determining who may participate in research and under what conditions;
- Providing consistent messaging for both researchers and participants on keeping oneself and others safe.
Our ongoing aims are to:
- Determine best practices for maintaining the health and safety of all research participants as we undertake a return to research on campus;
- Develop guidelines and requirements for ramping up in-person research;
- Identify practical ways and means for researchers to comply with in-person data collection guidelines (such as training, certifications, access to materials, oversight, consultation);
- Establish iterative phases with different densities of occupancy of research buildings, based on public health data;
- Act, alongside the Institutional Review Board, as the primary point of contact for assistance, guidance, and support concerning the research ramp-up process, regulatory and administrative guidance, and inquiries about research status.
To support these aims, the Research Ramp-Up Task Force is comprised of or working closely with personnel from the Provost’s Office, the Office of Finance and Administration, the Environmental Health and Safety Office, Public Safety, Facilities, the Institutional Review Board Office, the TC Information Technologies Office, the Office of Sponsored Programs, the Office of Risk Management, the Office of Special Projects, and the other campus-reopening committee groups.
Although we are being guided by parallel considerations at Columbia University and other institutions in the City, Teachers College is developing its own policies and procedures for our inperson research ramp-up initiative that are tailored to the needs of our researchers. Our aim is to clearly communicate policies and procedures and what next steps mean for you and your research. Please do not misinterpret delayed communication for inaction; rather, we are working to remain thoughtful and thorough in how we approach any situation – precisely because the stakes are so high.

Even after we start ramping up on-campus research, if you can do your work online, we encourage you to continue to work remotely. By keeping population density on campus as low as possible, we will be better able to protect our community and those who will start to come back to campus.
We will continue to inform the TC Research Community of our progress through email updates or postings on the Updates section of the TC IRB’s website.
We are very grateful to TC researchers and all those who are contributing to campus reopening efforts; the problems we face are multi-faceted, and every part of our community is engaged in addressing them. We look forward to our next steps back from the restrictions of these past months, and we thank you for your patience and continued flexibility.
Best regards,
Dr. Karen Froud & Dr. Myra Luna Lucero
On Campus Research Ramp Up Planning Committee
Dear Teachers College Researchers and Personnel:
As you know, the novel coronavirus (COVID-19) pandemic has created significant campus changes. Your research labs and centers have been on pause, and we all braced for short and long-term COVID-19 disruptions. Some of you have transitioned inperson data collection to online platforms, while others have paused in-person data collection indefinitely.
Due to COVID-19, all research-related activities taking place on campus in-person at Teachers College are still suspended.
However, we are all aware that some research studies cannot sustain long-term in-person data collection suspensions. For many researchers a key concern is fulfilling commitments (e.g., pursuit of knowledge, publications, grants, tenure, graduation, etc.). We are writing as Co-Chairs of the newly-formed Teachers College Research Ramp-Up Task Force, to let you know about the aims of the Task Force and upcoming plans. We aim to respond to the needs of researchers while minimizing risk to participants and staff.
Co-Chairs:
- Dr. Karen Froud, Institutional Review Board (IRB) Chair, Associate Professor of Neuroscience & Education
- Dr. Myra Luna Lucero, Research Compliance Manager
Manager Members:
- Dr. Stephanie Rowley, Provost
- JoAnne Williams, Vice President for Finance and Administration
- Katie Schmitt, Director of Special Projects
- Paul Kran, Office of Sponsored Programs Director
- John Olsen, Maintenance Project Manager, Facilities
The goal of the Task Force is to harness content-specific expertise and establish a plan for in-person research ramp-up at Teachers College with different phases that permit occupancy to be scaled depending on the scenario, personnel, and study site. Each phase should be adaptable as appropriate for the circumstances pertaining to specific research groups and risk levels.
As the Teachers College Research Ramp-Up Task Force, we will:
- Consider the safety and health of research participants, including research subjects, investigators, staff, and students as our main concern
- Remain accountable to the research community at Teachers College
- Keep open channels of communication to disseminate information in a timely and consistent manner
- Develop guides, policies, and procedures with an eye to their feasibility and accessibility
- Strive to develop approaches that are fair and flexible, and remain responsive to the uncertainties inherent in our current situation and the need for guidance and clarity
- Make evidence-based and data-driven decisions, based on information from reputable, expert sources and from the needs and experiences of our researchers
- Operate on the tenets of the Belmont Report, instantiating respect, beneficence, and justice for all research subjects, investigators, staff and students
- Recognize limitations and unknown factors
- Acknowledge that decisions may change due to new or relevant information

At present, we are gathering data to determine the scope of our initiatives. We are not lifting any in-person data collection suspensions. All in-person, research-related activities will remain suspended, until further notice.
The timeline for a return to in-person research remains unclear, though the “Safer-at-Home” mandate is current through August 1, 2020. We recognize anything can happen between now and August. However, we are using the August date as a marker to implement guidelines for an initial In-Person Research Ramp-Up phase.
We aim to support researchers through the many hurdles in preparation for eventual in-person data collection. Changes or guidelines to in-person data collection will follow an iterative process. Each phase will require a feedback period as we refine and scale-up researcher guidelines. In other words, no changes will be made in haste and your patience through this process is essential. We are taking care to weigh decisions and assess risks for both researchers and research participants. In addition to our Task Force members, we are also consulting with campus staff and researchers familiar with safety and public health.
Immediate Goals
These broad goals are subject to change. We aim to:
- Determine best practices for maintaining the health and safety of all research participants as we undertake a return to research on campus
- Develop guidelines and requirements for ramping up in-person research Identify practical ways and means for researchers to comply with inperson data collection guidelines (such as training, certifications, access to materials, oversight, consultation)
- Establish iterative phases with different densities of occupancy of research buildings, based on public health data
- Act, alongside the Institutional Review Board, as the primary point of contact for assistance, guidance, and support concerning the research ramp-up process, regulatory and administrative guidance, and inquiries about research status
In Summary
We will inform the TC Research Community of our progress through email updates or postings on the Updates section of the TC IRB’s website.
We wish to acknowledge that even though halting research on campus constituted an unprecedented professional challenge to our ongoing work, we have heard many accounts of creativity and persistence that mean our research has been able to continue in many different forms. We thank you for your remarkable flexibility and perseverance, and wish you all continued health, safety, and productivity.
Best regards,
Dr. Karen Froud & Dr. Myra Luna Lucero
TC IRB and Research Amid COVID-19
During the College closure, TC IRB operations will continue as normal. We will review all protocols as they come in, even protocols that cannot begin until the end of COVID-19 quarantine.
Protocols that include in-person procedures will be approved with restrictions on all or some activities until the time normal activities may resume. Once the college reopens, the TC IRB will issue guidance on when and under what circumstances in-person procedures may resume, and any safety measures deemed appropriate for in-person research. In no case should in-person research begin without express approval from TC IRB. We are closely monitoring updates from the Centers for Disease Control and Prevention (CDC) and campus officials. TC IRB will alert researchers of any study-related information as it becomes available. Please check TC IRB's website/Updates for current information.
In such a fluid situation, the IRB understands that certain sites may allow data collection before Teachers College reopens. If that is the case for your research please contact the IRB through "messages" in Mentor IRB or email IRB@tc.edu. TC IRB expects to allow such research to move forward if the external site allows it, but we need to consider each protocol individually to assess participant risk. TC IRB approval to resume in-person procedures at sites that require overnight travel will still be subject to any restrictions on College related travel. As of April 1, 2020, all such travel is suspended.
Overall Guidelines
TC IRB Business as Usual:
- TC IRB is reviewing protocol submissions as usual with some restrictions to in-person research
Online Studies:
- Online studies can begin immediately after TC IRB issues an approval letter
In-Person Studies:
- In-person research cannot begin until the COVID-19 quarantine is lifted and TC IRB issues a formal approval for in-person research to begin
- In-person studies will be reviewed and approved, with restrictions
Mixed Methods Research:
- For mixed methods research, the online portion may begin after IRB issues an approval letter, and the in-person portion will be approved, with restrictions
Questions?
- Use the How to Access Messages in TC Mentor IRB to locate "messages" in Mentor IRB
- Send questions to IRB@tc.edu or for a submitted protocol use "messages" in Mentor IRB
Continuing Research: COVID-19 UPDATED
Teachers College Institutional Review Board (IRB)
MEMORANDUM
DATE: March 16th, 2020
TO: IRB Protocol Principal Investigators, Study Coordinators, Research Staff
RE: Suspension of onsite, in-person research activities at Teachers College
As of March 16th, 2020, this memo outlines the Teachers College response to the Columbia mandate for a research ramp-down plan and implementation, dated March 15th, 2020.
In the spirit of collaboration with directives now in place at Columbia University, and with the safety of our investigators and research participants always at the forefront of our endeavors, all research-related activities taking place on campus in-person at Teachers College are suspended, with immediate effect.
You must immediately inform all members of your research team that on-site research activities at Teachers College are suspended, and that they may not schedule, attend, or carry out any research activities with an in-person component on the Teachers College campus.
Current telephone or on-line study activities, or remote data collection, may continue without interruption (unless directed otherwise by an external site). Check with your external site for next steps. NOTE: As of the time of writing, NYC Department of Education (DOE) schools are closed: https://www.nytimes.com/2020/03/15/nyregion/nyc-schools-closed.html
Study-related activities that do not require face-to-face interaction, such as data analysis, may continue off-campus. Such data analysis activities may only move off-campus if the following requirements are met:
- All identifiers are removed from any digital data (such as computer files, recordings) or physical data (such as paper records) that are taken off-campus and securely stored; AND
- Digital data protection procedures outlined in the approved study IRB protocol are maintained.
Please review TC IRB’s Data Security Plan for a guide on transferring, securing, and managing digital data. For more information about data security you can also visit: Data Sharing, Requests, & Encryption
Alternatives to face-to-face study activities may include telephone or teleconference (e.g., Skype or Zoom) sessions. You can access Zoom through MyTC. For more information about Zoom you can visit: https://teacherscollege.screenstepslive.com/s/14864/m/72448/l/1188775-recording-audio-with-zoom
There is no regulatory requirement to submit an IRB Modification to pause research protocols or activities; that is, protocols or components of protocols may be paused without a formal protocol modification process.
However, in response to COVID-19, we do require investigators who currently conduct in-person, on-campus research activities to indicate cooperation with this directive via email, as described below. Please read these directions carefully as this email confirmation only applies to investigators who conduct in-person, on-campus research.
FOR INVESTIGATORS WITH IN-PERSON, ON-CAMPUS RESEARCH ONLY, EMAIL IRB@tc.edu
To Pause your Research in Response to COVID-19:
Please email IRB@tc.edu to confirm that all in-person study activities intended to occur on Teachers College campus are paused (as they cannot be moved online through a COVID-19 modification).
When you email IRB@tc.edu include the header, "PAUSING IN-PERSON, ON-CAMPUS RESEARCH IN RESPONSE TO COVID-19." Also, include your IRB protocol number and name of the primary investigator in the body of your email. To find your IRB protocol number visit Mentor IRB (MyTC/Resources/Mentor IRB), as each protocol has a unique numeric identifier. Please see the example below:![]()
No further action will be required on your part after you send the email to IRB@tc.edu to pause your in-person, on-campus research in response to COVID-19. It is important to send this email confirming that you have paused your on-campus, face-to-face study activities no later than Wednesday, March 18, 2020. This email action will constitute your acknowledgement and confirmation as a principal investigator that your face-to-face, in-person, on-campus research activities are paused in compliance with this COVID-19 directive.
This request is only for researchers pausing on-campus, face-to-face study activities in response to COVID-19. If you are pausing your research for other purposes (e.g., timeline adjustments or typical research plans), you do not need to send an email to IRB@tc.edu.
- Please review the How to Submit COVID-19 Modification Online Activities walkthrough guide if you can move your research to online methods.
Appeal for In-Person Continuation:
We do understand that some research may be deemed essential, may carry direct benefits to participants, or may need to adhere to strict deadlines. In such cases, investigators may feel a need to continue in-person activities. However, at this time the IRB will make the final determination about in-person research that may be considered essential and allowed to carry on as planned.
If there are specific, extenuating circumstances that you believe constitute grounds for continuing in-person study activities contrary to this directive, please indicate this information in Mentor for IRB review and determination.
Please navigate to your protocol page in Mentor IRB. Click on the Modifications tab and select “Create New Modification.” Check the box next to "My Study Methods Cannot be Moved Online for Covid-19 and Study Cannot be Paused" and then, when the modification is created, click on the link to the "My Study Cannot be Moved Online" (links in Mentor usually appear as dark red text). This will take you to a short survey to provide more information as to why your study should continue with direct face-to-face research participant contact.
TC IRB will make the final determination on your modification submission. Please note that, even if your research is approved to continue at this time, ONLY 2-4 ESSENTIAL PERSONNEL per research study may be permitted to work on campus, requiring a significant curtailment of in-person research activities. IRB decisions about such requests will be made as promptly as possible.
- Please review the How to Submit COVID-19 Modification Online Activities walkthrough guide for moving your research to online methods.
- Please review the How to Submit COVID-19 Modification Studies Not Online walkthrough guide for studies that cannot moved online.
- We also have a walkthrough guide for instances when some of your research activities must remain in-person, yet others can be moved online. In these cases, please review the How to Submit COVID-19 Modifications for Hybrid Activities walkthrough guide.
In-Person Research Activities at External Sites (not at Teachers College campus):
If your research is taking place at a site other than Teachers College, and no directive from that site has been issued to suspend in-person activities, your research may continue. However, we ask each investigator at this time to carefully consider the risk/benefit ratio associated with such continuations, and we strongly recommend that in-person research at external sites is also shifted to online formats or paused.
Placing investigators or subjects at greater risk to continue with research activities is unacceptable. Please use your ethical compass to determine the best path forward for your research protocol and the protection of research participants at external sites. This may mean communicating with the external site IRB or TC IRB on next steps, moving study activities online, or pausing in-person study activities. Researchers can utilize TC IRB’s “COVID-19 study modification” options in Mentor IRB to communicate protocol changes. Please review the Response to COVID-19 walkthrough guides for IRB protocol Modification options.
- Please review the How to Submit COVID-19 Modification Online Activities walkthrough guide for moving your research to online methods.
Researchers may also want to review the International Compilation of Human Research Standards for information about research abroad.
For Researchers Moving to Online Methods:
If you are not suspending your study but moving to online methods, please navigate to your protocol page in Mentor IRB. Click on the Modifications tab and click "Create New Modification." Check the box next to "Moving to Online Methods in Response to Covid-19 (no further approval needed)" and then, when the modification is created, click on the link to the "Moving Study Online Questions" (links in Mentor usually appear as dark red text). This will take you to a short survey to provide more information.
Please note that you do not need to wait for IRB approval if you are moving your study procedures online, though all modifications will be reviewed in due course.
- Please review the How to Submit COVID-19 Modification Online Activities walkthrough guide for moving your research to online methods.
Resuming In-Person, On-Campus Activities:
Resuming in-person research protocols on campus, considering COVID-19, will first need TC IRB review and approval, and approval by Teachers College campus officials. The IRB must be notified via email (IRB@tc.edu) when you plan to resume in-person, on-campus study activities. In the email header state, "RESUMING IN-PERSON, ON-CAMPUS RESEARCH POST-COVID-19." In the body of the email, include your IRB protocol number, name of the primary investigator, and the exact date you plan to resume in-person, on-campus study activities. TC IRB will review current COVID-19 reports and if applicable provide formal permission for the primary investigator to resume in-person study activities on campus.
Because all research-related activities taking place on campus in-person at Teachers College are suspended, you may not resume your in-person study activities on campus without TC IRB permission. We anticipate that this period of in-person, on-campus suspension will last at least 6-8 weeks, as the situation is still dynamic. Announcements will be made prior to any resumption of research activities on campus. No resumption of in-person, on-campus activities is permitted without TC IRB review and approval.
As always, we thank all our investigators and research staff for their flexibility and professionalism under these trying circumstances. We do understand that research is central to the mission of the College and to each individual's work. We have been unwilling to impose these study activity limitations but we also recognize that ethical principles of research and federal regulations for the protection of human research participants require an acceptable risk/benefit ratio. Under current circumstances, the risk/benefit ratio for research participation is deemed to have shifted significantly.
We do ask that you limit direct inquiries to the IRB Office at this time as we are handling more than our usual volume of inquiries. Be assured that TC IRB will continue to monitor the evolving COVID-19 outbreak and communicate best practices to our investigators. We will post any further updates on TC IRB's webpage/Updates.
Stay safe and well.
Dr. Karen Froud, IRB Chair
Dr. Myra Luna Lucero, IRB Compliance Manager
Continuing Research: COVID-19 Update
As of March 10, 2020, the Teachers College (TC) Institutional Review Board (IRB) continues to monitor the evolving COVID-19 situation. We are currently evaluating how human subjects research will be impacted due to COVID-19. We are aware that the TC campus will switch to a largely online environment for the next several days. At present, this directive does not apply to research activities on campus.
However, we urge all researchers to consider the potential effects of continuing to invite research participants to campus. In some cases, researchers may be able to move face-to-face contact with research subjects online (e.g., Zoom/Skype). In other cases, the study procedures will be such that online substitutes are not practical or even possible (for example, research projects on a tight deadline for completion; or where there is potential harm to participants if study procedures stop).
We have implemented new Mentor IRB modification submission options in response to COVID-19. (For a guide on how to submit a regular study modification, please visit our Modification page). Please submit a COVID-19 modification only if your study is impacted by COVID-19. All other study activities not impacted by COVID-19 should be addressed through regular channels (e.g., typical modification).
The COVID-19-specific modifications through Mentor IRB include:
- Moving Research Methods Online Modification: If it is feasible to conduct some or all the research methods online, and/or if the study site(s) have closed due to COVID-19 precautions, researchers can select the Mentor IRB Modification type: “Research Site Closed for COVID-19 - Moving to Online Methods”
- Research Methods Cannot Be Moved Online or Paused Modification: If it is necessary to continue this research study in person with the research subjects due to the nature of the study procedures, and/or there are possible risks to subjects if the study were to be paused, researchers can select the Mentor IRB Modification type: “My Study Methods Cannot be Moved Online for COVID-19 and Study Cannot be Paused”
- Some Methods Can Be Moved Online While Others Cannot: In some cases, some study activities in a protocol may be transferred online, while other activities in the same protocol are not substitutable. If it is necessary to continue some of the research activities in person and have other activities are moved online, researchers should select both Mentor IRB modification types: “Research Site Closed for COVID-19 - Moving to Online Methods” AND “My Study Methods Cannot be Moved Online for COVID-19 and Study Cannot be Paused”
NOTE: Unless research subjects would be placed at risk by pausing/discontinuing the study temporarily, researchers may pause work without filing any Modification to an IRB approved study.
The IRB may also direct researchers to stop or pause a study. If this happens, we will contact researchers with instructions on next steps.
Submitting COVID-19-Specific Modifications
Step-by-step directions for submitting COVID-19-specific modifications through Mentor IRB are available here:
- Moving Research Methods Online Modification: How to Submit COVID-19 Modification Online Activities
- Research Methods Cannot Be Moved Online or Paused Modification: How to Submit COVID-19 Modification Studies Not Online
- Some Methods Can Be Moved Online While Others Cannot: How to Submit COVID-19 Modifications for Hybrid Activities
Continuing On-Campus Research
If you decide to continue on-campus research work, please follow common sense precautions for the protection of participants and researchers. Suggestions include:
- Washing hands frequently, for at least 20 seconds using soap and water each time, and/or using hand sanitizer in between contact with each research participant
- Using disinfectant wipes down all flat surfaces, including desks, chairs, door handles, and any equipment that is in contact with participants, between each use
- Wearing gloves as much as possible, especially for hands-on activities such as electrode placement
- Avoid gathering large groups of participants together in one place
- Maintaining "social distancing" recommendations as determined by the CDC: "Social distancing means remaining out of congregate settings, avoiding mass gatherings, and maintaining distance (approximately 6 feet or 2 meters) from others when possible" https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html
- When inviting participants to the College, ask them to please stay away if they are experiencing any respiratory illness, coughing, or sneezing
- When escorting research participants through the College to your Lab or Center, follow the same route each time, avoid crowded places, and alert campus security or custodial if you require any additional assistance
For research happening at offsite locations, we recommend implementing similar strategies as appropriate, and following the guidelines of the specific research site. Please defer to the site-specific recommendations and directives for the continuation or cessation of research activities.
Thank you for your continued patience, flexibility, and professionalism during this complicated time. and be assured that TC IRB will continue to monitor the evolving COVID-19 outbreak and communicate best practices to our investigators.
Travel Preparedness
March 2020
For information on travel preparedness and advisory updates, please visit TC and Columbia University's websites.
Empower Hour: Topic Driven Seminars
February 2020
Join Dr. Myra Luna Lucero as she presents insightful seminars on the inner workings of things that are expected in the classroom but are not directly discussed. These bi-monthly topic driven seminars aim to empower students and faculty to take charge of their research. RSVP for any or all of the seminars at http://bit.ly/empowerhour20
| Date | Topic |
|---|---|
| February 11 | Demystifying the IRB |
| February 18 | Conference Submissions & Presentations |
| March 10 | IRB Proposal Review |
| March 31 | IRB Proposal Editors Circle |
| April 14 | Grant Funding Your Academic Research 101 |
| April 28 | What Does It Mean to be a Good Mentor/Mentee? |
All seminars will be held on Tuesdays, from 3:00 to 4:30 PM.
Presented in affiliation with Office of Sponsored Programs, Student Affairs, Student Senate, and ODCA's Diversity in Doctoral Education Initiative.
Required Updates to CITI Training
Researchers working with human subjects may not begin recruitment or research until they receive a final IRB approval letter. All research staff and faculty sponsors submitting an IRB protocol must have an updated CITI training certificate awarded within the last three years filed in Mentor IRB/PI Documentation. Researchers who were trained in offline settings are required to complete the full basic online course through CITI's website. Researchers who completed the Basic Course online through CITI can complete the refresher course. An updated CITI training certificate (awarded within the last three years) is required for all IRB submissions. Please visit our Training & Certification page for information on how to update CITI certification.

Federal Regulations - Common Rule
January 2019
The new federal regulations covering research with human subjects (aka the New Common Rule) have been in effect since mid-January 2019. The following is an update on our implementation efforts, and other ongoing efforts to ease the administrative burden on the Primary Investigator (PI).
- Continuing Review: Continuing review is no longer required for expedited protocols. In the months leading up to the switchover to the new regulations, the IRB office became aware of a large number of ongoing studies that were not using the IRB approved stamped consent form. In light of that finding, the IRB Full Board voted to require an annual “check-in”, an abbreviated form that asks if the study is still ongoing and to upload the consent forms the project is currently using. Work with subjects on expedited protocols can continue uninterrupted through this process since IRB approval is good until you terminate the study.
- Protocol Terminations: A study can be closed once it is closed to recruitment and all data collection has concluded. Data analysis can continue after the protocol is terminated. Researchers can also receive follow-up clinical data from healthcare providers after the IRB protocol is terminated.
- Update Expired Human Subject Training by February 1, 2020: Implementation of the three-year renewal of IRB training has been delayed. We will now require all PIs who have not taken the CITI training within the past three years to complete the refresher courses by February 1, 2020. Please visit our Training & Certification page for more information on how to renew your CITI training.
- Faculty and professional staff PIs will receive notification from the IRB office that their training has expired.
- Since many student PIs will have left the college three years after completing the CITI training, we will notify any students with expired training at the time of protocol submission.
- In the Interim, the Office of Sponsored Program (OSP) will ask any PI who receives a new federal award and their project personnel to take the CITI refresher course during the “Just-in-Time” period after the award notice and the official final award. Anyone who needs to take the refresher course in order to comply with the New York City Department of Education (NYC DOE) training requirements should contact IRB@tc.edu or 212-678-4105.
- TC IRB’s Standard Operating Procedures: TC IRB has an updated set of Standard Operating Procedures posted to Mentor and available for download. We encourage researchers to review it prior to submission to the TC IRB, but we expect most researchers will use it as a reference when questions arise about TC IRB policies and procedures in response to reviewer feedback.
- NYC DOE Consent Form Template(s): In order to facilitate research within NYC public schools, TC has posted NYC Department of Education’s preferred informed consent template(s) to Mentor/Documentation. Soon, if you check “yes” to the question, “Does this research take place in NYC public schools?” in Mentor’s PI Survey, you will be instantly directed to the DOE’s consent form template(s). TC IRB will accept DOE consent form templates in place of our own standard templates. The use of DOE IRB’s templates should make submissions to the DOE’s IRB go far more smoothly.
- Dynamic forms in Mentor: We have implemented Mentor’s dynamic form system for exempt review categories. When you request an exemption under one of the exempt categories, you will be directed to a set of questions specific to that particular exemption. The amount of information the IRB needs to make an exemption determination is far less than what it needs to review expedited and full committee IRB protocols. Since nearly half the protocols submitted to the IRB are exempt, this will be a considerable time-saver. Over the summer, we will be trying out and piloting new dynamic forms for nonexempt research to see if we can streamline the application process more generally.
Finally, just a reminder that TC IRB is now a member at Research Match, a database that connects people interested in participating in research with PIs.