Conducting ethical research includes a well-developed consenting process with human subjects. Consent processes may vary, but they always include: (1) information disclosure; (2) assessment of competency to consent and the participants’ ability to make a decision, and (3) emphasize the voluntary nature of the decision to participate in research. This blog post presents researchers with expectations for the consenting process.
What are the legal requirements for Informed Consent?
For consent to be ethically and legally valid, it must meet the requirements stated in Principle I of the Federal Regulations (45 CFR 46:116) which is based, in part, upon the Nuremberg Code. Principle I of the Nuremberg Code states,
“The voluntary consent of the human subject is essential. This means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over-reaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved as to enable him to make an understanding and enlightened decision.”
The ethical and legal validity of volunteering for a study is dependent upon the consent process which requires dialogue, information sharing, or negotiation between researcher and prospective study participant. The consent form is an instrument to guide the consent process.
Researchers are responsible for developing consent forms for information-sharing that emphasize the voluntary nature of study participation. The consenting process includes a researcher's assessment of the cognitive competency of the prospective participant to make a decision and includes documentation of the individuals’ decision to agree to volunteer for the study (Kodama Muscente, 2022).
Vulnerable populations are subject to a higher possibility of coercion or undue influence and therefore require researchers to prepare additional safeguards during the consenting process and study procedures.
What are the general requirements for Informed Consent?
Informed consent is more than just a signature or single verbal affirmation that an individual wants to participate in a research study. It is a process, built on trust and respect, spread throughout a study. The process often begins with the initial signature or affirmation of consent but may often also include continued check-ins with the participant to confirm consistent willingness to participate and to provide them with the opportunity for questions, and concerns, and to stop, should they wish.
The process of obtaining informed consent begins before data collection and any other research procedure (i.e., intervention). Typically, this consent form process starts with opening a discussion with the participant about what participation in the study entails, establishing rapport, and documenting the participant’s agreement to continue with the study procedures.
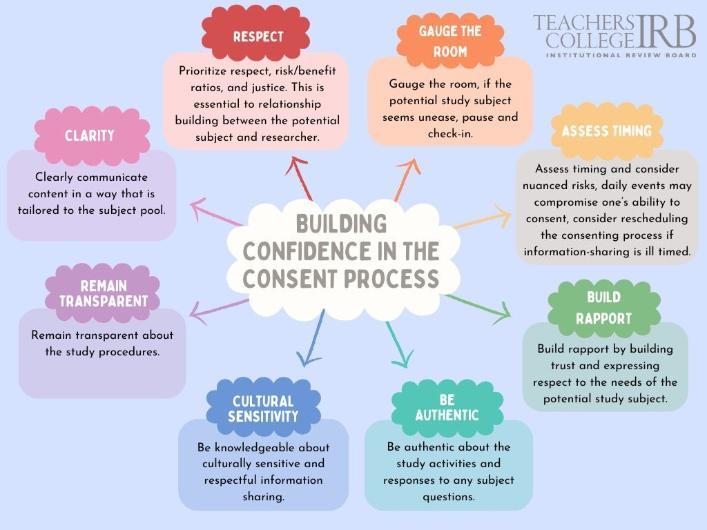
An informed consent process is more than just handing an information sheet to potential study subjects and asking for their signature. The consent process is an open and ongoing communication exchange between the researcher and the individual, free of coercion, and bias, and with an emphasis on volunteerism. Researchers’ confidence impacts the consenting process. To prepare, researchers should, (1) confidently know and understand the study activities, (2) prepare for common questions that may arise about the study, (3) leverage the use of visual aids and other supplementary materials to explain complex study procedures or concepts in simple terms; (4) allocate sufficient time to respond to any question, and (5) be knowledgeable about disseminating information in a way that is culturally sensitive and respectful of population pool.
Emphasize Volunteerism, Strive for Readability and Understanding
An individual is never obligated to participate in a research study, they choose to volunteer of their own free will. The consent process is designed to share information in a way that is understandable for the population of interest. All recruitment and consent materials should contain thoughtfully chosen language to facilitate a thorough understanding of the study procedures, purpose, risks, and any possible benefits.
Choosing more common words or phrases while presenting study information is always more appropriate than academic wording as you can never guarantee these words are recognizable to your population of interest. Keep in mind that even if the target audience for your consent materials is average adults, reading levels may vary as well. When writing for the average adult participant, it is best practice to aim for an 8th-grade reading level. One way to check the reading level of your documents is to utilize the Flesch-Kincaid Grade Level and Reading Ease formula:
This formula may help aid in determining the level of readability of your participant documents. Keep in mind, however, that this formula is best suited for determining appropriate sentence length. Other factors that contribute to ease of readability, such as sentence structure, word variety, sentence clarity, and difficulty of vocabulary would need to be separately considered.
Additionally, exculpatory language is never acceptable in any consent forms. Exculpatory language is any language that waives or appears to waive any of the participant’s legal rights or the rights of the participant’s representative.
Examples of Exculpatory language include (Health and Human Services, 2011):
- “I waive any possibility of compensation, including any right to sue, for injuries that I may receive as a result of participation in this research.
- If you suffer a research-related injury, neither the institution nor the investigator can assume financial responsibility or liability for the expenses of treatment for such injury.
- If you suffer a research-related injury, your medical expenses will be your responsibility or that of your third-party payer.”
All consent materials must comply with both the legal and ethical requirements to obtain informed consent to be approved by TC IRB for use in human subjects research.
To summarize:
At a minimum, a consent form should have the following information:
- Purpose of the study
- Activities, tasks, duration, risks, and benefits
- Describe the types of activities and tasks the participant may complete
- Survey, interview, observation, etc.
- Voluntary-participation clause
- Participants need to be made aware that they do not have to participate in the study, and that it is fully voluntary. If they decide not to participate they must be informed that it will not affect any relationships they have with the researcher and/or facility in which it is administered.
- The consent should also make it clear to participants how they can withdraw from the study at any point without penalty.
- Participant-data handling
- How will personal data, findings, and recordings (if applicable) be handled?
- Will data be anonymized? If so, how much of it will be anonymized?
- How will it be used, accessed, secured, maintained, and stored? Who has access to this information?
- When will the raw, recorded data be deleted (if ever), and by when?
- Where will it ever be published?
- Consent statement
- “I voluntarily agree to participate in this research program.”
- I agree to the terms of this study and consent to participate.
- I consent to audio being recorded during the session for analysis purposes.
- I consent to the video being recorded during the session for analysis purposes.
- Compensation (if applicable)
- If compensation for the participants’ time, be it monetary or otherwise state the details in the consent form.
- Compensation is not based on the participants’ abilities.
- Study contact & support
- Participants should be informed about the support available to them during the study, such as contacts for questions about their rights, whom to reach out to for study-related concerns, and any counseling or support services if the research could potentially cause distress.
- Information (or debrief) sheet (as needed)
- Depending on the study design, additional information may include a debriefing script, an additional study activity information sheet to describe in detail the tools or materials used, or any other information to clarify the study.
For more resources on Informed consent, check out our blog posts and guides:
- Aristotle & Consent as a Process
- Be Informed about Consenting
- Prepare, present and polish the informed consent form
- Assessing Potential Research Participant’s Competency to Consent
- Writing for an IRB Review
- Notes on Recruitment Materials
Resources for the Flesch Reading Ease Readability formula
- “How to” for Microsoft Word
- Google Docs does not have the feature to calculate the formula without a third party application. Some that you can use are:
